Determination of Some Nutritional Qualities of Solanum aethiopicum (Eggplant) Fruit Flours Obtained at Different Temperatures
Kaana Asemave*, Ungwanen Ahile and Oscar Terungwa Alhaji
Department Chemistry, Benue State University, Makurdi, Nigeria
Received Date: 18/12/2022; Published Date: 06/01/2023
*Corresponding author: Kaana Asemave, Department Chemistry, Benue State University, Makurdi, Nigeria
Abstract
Processing eggplant fruit into flour will help to minimize post-harvest losses, therefore enhancing its use as food. Hence, the work was aimed at determination of some nutritional properties of eggplant fruit flour. Eggplant fruits purchased from Gboko Market, Benue-Nigeria were washed thoroughly, sliced, and dried at 45 for oven drying and 55 for tunnel drying. Each of the dried slices were ground into powder and packed into plastic bags for analysis. The flours dried at 45 and 55 were then designated as A and B, respectively. Standard laboratory methods were used for the analyses. The results showed that the moisture and ash levels of A and B were not significantly different, unlike the other proximate parameters. In addition, A had 89.1 0.01 mg/100 g flavonoid, while B gave 63.5 0.01 mg/100 g. 263.1 0.01 mg/100 g and 249.7 0.02 mg/100 g alkaloids were obtained in A and B, respectively. Total phenols in A were 42.30 0.01 mg/100 g and 43.2 0.03 mg/100 g for B. Flour A gave 347.4 0.01 mg/100 g anthocyanins and B had 281.3 0.02 mg/100 g. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) inhibition (%) of 379.4 0.01 was found in A and 363.2 0.01 for B. Of the minerals, Na, K, P, Zn, and Mg; Zn was the most present with levels of 2.04 0.00 mg/100 g in A and 2.02 0.00 mg/100 g for B; whereas, P recorded least amount. Furthermore, conductivities observed were 515.3 0.58 µs/cm (A) and 512.3 1.53 µs/cm (B). A had water absorption capacity (WAC) of 3.41 0.02%, while B gave 3.28 0.65%. Swelling capacity of A and B were 1.74 0.01 mL and 1.52 0.00 mL, respectively. Meanwhile, the total dissolved solids (TDS) found were 249.6 0.58 mg/L (for A) and 214.6 1.15 mg/L (for B). For bulk density, A gave 0.29 0.00 g/cm3 and 0.22 0.00 g/cm3 was obtained for B. Values of 4.16 (in A) and 4.17 (for B) were detected as the pH; while titratable acidity indicated 0.44 0.01 g/L and 0.23 0.22 g/L for A and B, respectively. Thus, the eggplant fruit flour is full of bioactive compounds, minerals and functional properties.
Keywords: Eggplant Fruit Flour; Nutritional and Functional qualities; Bioactive compounds
Introduction
Epidemiological, toxicological, and nutritional studies have proven that coronary heart problems, cancer, diabetes, and Alzheimer’s disease can be minimized with consumption of fruit and vegetable [1]. Similar findings have also been described by Rodriguez-Jimenez et al. [2] that there is a positive correlation between the regular intake of phytochemicals and the prevention of lifestyle-related diseases [2-5]. Therefore, nowadays, the food industry has not only focused in the development of products with requisite nutrients for human food, but also for the prevention of diseases like diabetes, obesity, hypertension, and cardiovascular issues [2,6]. Eggplant is an economically and nutritionally important vegetable crop that is suitably grown in tropical and sub-tropical areas [2,7]. It is one of the most consumed vegetables due to its excellent nutritional value and antioxidant action [2,8,9]. The global annual production of eggplant is about 50 million tons [10]. Like other fruits, its consumption is steadily increasing because of more awareness of the health benefits associated with constant consumption of fruits and vegetables [11].
Typically, it composes of elevated amounts of vitamin A, folate, vitamin K, vitamin C, K, P, Mg and Ca [9,10]. Phenolic compounds and anthocyanins - with antioxidant functions - are also common in eggplant. In addition, the phenolic compounds minimize/regulate absorption of glucose to the advantage of patients with diabetes mellitus. Inhibition of lipid peroxidation is ensured by the anthocyanins; thus, the anthocyanins help with prevention of hyperlipidemia atherogenic cardiovascular disease [10]. Furthermore, eggplant is also rich source of various essential compounds like aspartic acid, tropane, flavonoids, lanosterol, gramisterol, steroid alkaloids, glycoalkaloids, histidine, nasunin, oxalic acid, solasodine, ascorbic acid and tryptophan, and vitamins that help in keeping good health [9]. For example, a major phenolic compound chlorogenic acid (5-O-caffeoyl-quinic acid; CGA), found in eggplant work as an anti-obesity, anti-inflammatory, anti-diabetic agent and also have cardio-protective functions [3,9]. Chlorogenic acid also shows anticarcinogenic functions by making apoptosis in many human cancer cells, such as leukemia and lung cancer cells. Eggplant is also effective against bacteria such as Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Vibrio cholerae, Pseudomonas sp. and B. cereus [9]. More so, it has compounds that are helpful in the cure of various diseases like anti-asthmatic, anti-platelet hypo-lipidemic, hypotensive, burns, warts, gastritis, stomatitis and arthritis etc. little wonder, traditional herbal medicines and practices is till among us in many parts of the world [9]. Eggplants also has low caloric level, which could be used in weight reduction diets [10][9]. Moreover, they are also helpful in ulcer treatment and vision [9].
Despite all these benefits, the per capita consumption of eggplant is small and it is 0.88 lb/year [10]. The low acceptance is because of the presence of nicotinoid alkaloids that makes it bitter [10]. Meanwhile, the World WHO recommends 400 g/day of fruits and vegetables [10]. Therefore, due high nutritional and health benefits eggplant, eggplant flour has been introduced in common food products such as cookies, breads, cakes and pasta in general [10]. Eggplant with non-climacteric pattern of respiration has short shelf life even if harvested at an immature stage. During harvesting, eggplants suffer heavy losses due to oversupply [3]. Thus, it is better to process eggplants into flour to avoid losses and take advantage of their good nutritional profile [2]. Moreover, in tropical countries, postharvest infrastructure (cold storage facilities, refrigerated transport, packinghouses, etc.) are either scarce or not functioning properly. Long-term storage is not suitable for almost all kind of fruits and vegetables [7]. The recommended commercial storage for eggplant is set generally for less than 14 days at 10-12 and 90 to 95% relative humidity [7]. Hence, its flour may be prepared by a process of lyophilization that involves removing water from the food by sublimation at a high vacuum, however, the process is expensive. Alternatively, dehydration in oven seems to be more feasible. Moreover, when compared to lyophilization; drying increases the antioxidant capacity; by retaining more phenolic compounds, flavonoids, and anthocyanins in eggplants [10]. Therefore, the paper reports determination of some nutritional qualities of Solanum aethiopicum (Eggplant) flours obtained at different temperatures.
Materials and Methods
Sample Collection
Eggplant was purchased from the Gboko market, Benue State - Nigeria. They were fresh and free from physical damage. Purple colour and round shape. The eggplant fruits were identified as Solanum aethiopicum in the Department of Biological Science of Benue State University – Nigeria by a Botanist.
Sample Preparation
Thereafter, eggplant was washed thoroughly under running tap water to remove particles and then sliced with electrical stainless slicer so that they will be in uniform thickness and size. They were then at 45 in hot air over dryer and at 55oC in a tunnel dryer. The dried eggplants were pulverized into flour with using blender. The flour samples were packed into plastic bags, sealed and labelled. They were sealed properly to protect the samples. The dry temperature ranges were based on the past experience as reported in [10].
Determination of Proximate Composition
The analyses were performed according to the Association of Official Analytical Chemistry [12]. Ash, moisture, and crude fiber content were evaluated gravimetrically (method AOAC 14.006, AOAC 925.15, and AOAC 962.09, respectively). The Goldfisch method (AOAC 920.36C) was used to determine the fat content. The protein content was measured using the Kjeldahl method (AOAC 930.29), and total carbohydrates were determined by difference [2,13].
Determination of Physicochemical Parameters
Determination of pH
The method of the Association of Official Analytical Chemists (AOAC 1995) [14] was used for pH determination. 1 g sample was dissolved in 10 mL of distilled water in 100 mL beaker and mixed thoroughly; and the pH was measured with a pH meter.
Determination of titratable acidity (TA)
1 g of the processed ash sample was dissolved in 20 mL of distilled water in the conical flask, two drops of phenolphthalein indicator was added. A prepared solution of 0.1 M of sodium hydroxide was filled in the burette and titrated against the sample prepared in the flask until pink endpoint observed. The TA was calculated as: (H2Ta g/L) = 0.75 x Titer mL (of 0.10 M NaOH) [2].
Determination of phytochemicals
Determination of Total Flavonoid Content
Total flavonoid content was measured by the aluminium chloride colorimetric assay similarly described in [2]. the reaction mixture consisted of 1 mL of extract and 4 mL of distilled water was taken in a 10 mL volumetric flask. To the flask, 0.30 mL of 5% sodium nitrite was treated and after 5 min, 0.3 mL of 10% aluminum chloride was mixed. After 5 min, 2 mL of 1 M sodium hydroxide was treated and diluted to 10 mL with distilled water. A set of reference standard solution of quercetin (20, 40, 60, 80, and 100 ug/mL) were prepared in the same manner as described earlier. The absorbance for test and standard solutions was determined against the blank at 510 nm using UV/visible spectrophotometer (model 725S). The Total flavonoids content was expressed as mg of QE/100 g of flour.
Determination of Total Phenolic Content
Total Phenolic contents were determined by the Follin-ciocalteu method as described in [13][2]. About 0.02 mL aliquot of extract solution was mixed with 1.16 mL distilled water and 0.1 mL of Follin-ciocalteu reagent followed by addition of 0.3 mL of Na2CO3 solution (20%). Subsequently, the mixture was incubated in a shaking incubator at 40 for 30 min and its absorbance was measured at 760 nm using a spectrophotometer (Model 725S). Gallic acid was used as calibration standard, and the total phenolic content was expressed as gallic acid equivalent in mg/100g dry weight (mg GAE/100g DW)
Determination of Alkaloids
5 g of the sample was weighed into a 250 mL beaker and 200 mL of 10% acetic acid in ethanol added. The beaker was covered and allowed to stand for 4 h. It was then filtered and the extract concentrated on a water-bath to one-quarter of the original volume. Concentrated ammonium hydroxide was added dropwise to the extract until the precipitation was complete. The whole solution was allowed to settle and the precipitate was collected and washed with dilute ammonium hydroxide (2M) and then filtered. The residue if available, is the alkaloid which is then dried and weighed [15].
Determination of Anthocyanin
The total anthocyanins content was evaluated according to [2]. For the extraction of anthocyanins, 200 mg sample was mixed with 10 mL of ethanol-HCl 1N (85:15 v/v, pH 1, 4 ), purged for 30 sec with argon and stirred for 30 min at 200 rpm. Afterwards, the sample was centrifuged at 7759 g (4 , 15 min) and finally, 3.5 mL of sample was measured at 535 nm. The content of was reported as milligrams of cyanidin-3-glucoside (C3G) per kilogram of flour (mgC3GE/kg) as follows: C = (A/ε) (V/1000) MW (1/weight of sample) 106, where: C = concentration in mg C3GE/L, A = absorbance of sample, ε = molar absorptivity (mgC3GE = 26,965 cm-1mol‑1), V = volume of sample, and MW = molecular weight of C3G (449.2 g/mol).
DPPH Free Radical Scavenging Assay
9 separate 5 mL volumetric flasks were taken and aliquots of 0.1 mL, 0.5 mL, 1.0 mL, 1.5 mL, 2.0 mL, 2.5 mL, 3.0 mL, 4.0 mL and 4.5 mL of 1.0% of sample were added respectively to separate volumetric flasks. 0.5 mL of 0.2 mg/mL of DPPH was added to each of the mentioned 9 volumetric flasks. Volume was made up to the mark with ethanol, the flask was shaken vigorously and allowed to stand at room temperature, protected from light for 30 min. Absorbance was measured immediately at 517 nm by using UV spectrophotometer (Shinmadzu UV –Visible 160A) and experiment was done in triplicate. The IC50 value of the standard, which is the concentration of the standard required to inhibit 50% of the DPPH free radical was calculated using log dose inhibition curve. Lower absorbance of the reaction mixture indicated higher free radical activity [16]. The percentage of DPPH scavenging effect was calculated by following equation; DPPH scavenging effect (%) / % inhibition = A0 – A1/A0 x 100. A0 = absorbance of the control and A1 = the absorbance of the standard.
Mineral Content Determination
Mineral contents were determined as described in [17]. The samples were burned in a muffle furnace at 550 , then dissolved in 10% HCl. The solution was carefully filtered in a 100 mL volumetric flask and finally, distilled water was added to make up the mark. Thereafter, Na, K, Zn, mg, and p were determined using Atomic Absorption Spectrophotometer (Varian company USA).
Determination of the functional properties
Determination of bulk density (BD)
The volume of 10 g of the flour was measured in a measuring cylinder (15 mL) after tapping the cylinder on a wooden plank until no visible decrease in volume was noticed, and based on the weight and volume, the apparent (bulk) density was calculated [18].
Determination of swelling capacity (SC)
100 mL graduated cylinder was filled with the sample to 10 mL mark. The distilled water was added to give a total volume of 50 mL. The top of the graduated cylinder was tightly covered and mixed by inverting the cylinder. The suspension was inverted again after 2 min and left to stand for a further 8 min. The volume occupied by the sample was taken after the 8th min [18].
Determination of water absorption capacity (WAC)
1 g of sample mixed with 10 mL distilled water and allow to stand at ambient temperature (30 ± 2 °C) for 30 min, the centrifuged for 30 min at 3,000 rpm or 2000 × g. Water absorption was examined as % water bound per gram flour [18].
Determination of total dissolved solids (TDS)
Only 1 gram of sample was weight into 100 mL and 10 mL distilled water was added. The solution was mixed thoroughly on a magnetic stirrer for 2 h at ambient temperature of 32 . The solution was filtered on a screen filter paper into a cleaned grease free weighed Pyrex beaker. The filtrates were evaporated on a water bath, dried in the oven at 60 , was cool in desiccators avoiding stain and the beaker was reweighed. The gained in weight of beaker is equal to the total soluble solid per one gram of sample [19].
Results and Discussion
Proximate Composition
The proximate composition analysis results of the eggplant flours are in Table 1.
Table 1: Proximate Composition.
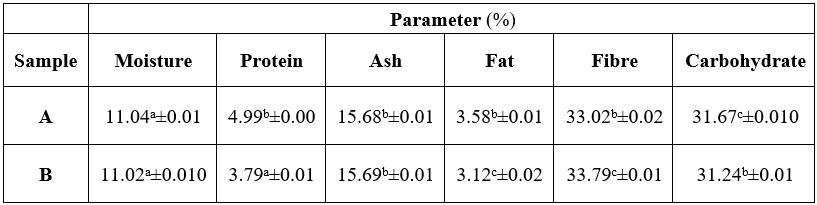
Key: A= Oven dried, B= Tunnel dry. Values are means of triplicate records. Means with different superscripts are significant at p > 0.05.
The moisture content of eggplant was in the range of 11.04% - 11.02%. The results showed that the moisture and ash levels of A and B were not significantly different, unlike the other proximate parameters. The % protein for these flours were 4.99 and 3.79 for A and B, respectively. A range values of 15.68 – 15.69% was found for the ash of these flours. Fat contents of the flours prepared at 45 and 55 were 3.58% (A) and 3.12% (B) respectively. Flours A and B gave fibre range of 33.02 – 33.79%. For the carbohydrate, the % values of 31.67 and 31.24 were observed for A and B, respectively. Agoreyo et al. [20] had reported proximate composition of Solanum melongena as 9.45%, 4.51%, 2.39%, 1.01%, 14.7% and 52.8% of moisture, protein, ash, fat, fibre and carbohydrate, respectively. Eggplant fruits have relatively higher carbohydrate (7.2 g/100g), crude fibres (2.0 g/100g), calcium (28 mg/100g), iron (1.5 mg/100), carotene (0.35 mg/100g) and ascorbic acid (8 mg/100g) than other fruits cultivated in the African countries [11]. Related to this finding, moisture, 5.5 g/100g; ash, 7.4 g/100g; protein 11.4 g/100g; lipid 2.1 g/100g; carbohydrate 39.1 g/100g; total fiber 34.5 g/100 g were previously reported [10]. Eggplant is one of the most commonly consumed vegetables in Côte d’Ivoire. The proximate composition in dry weight basis was significantly (P < 0.05) varied and ranged: dry matter 86.72-93.29%, crude protein 9.94-22.11%, crude fat 5.07-20.43%, crude fiber 23.15-50.53%, crude ash 5.14-10.09%, carbohydrate 12.41-39.22%, and energy value 211.53-320.94 kcal/100g [13]. Fiber contents present in eggplant helps in digestion by removing toxins and harmful materials from our stomach thus by reducing stomach and colon cancer [9].
Phytochemicals
Table 2 is the result for phytochemicals composition in the flours.
Table 2: Phytochemical Composition.
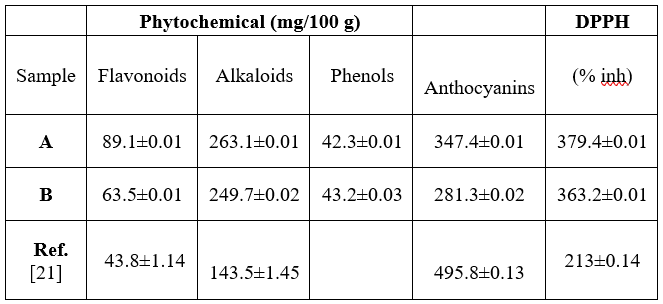
Key: Antho= Anthocyanins, DPPH (% inh) = 2,2-Diphenyl-1-picrylhydrazyl (% inhibition)
It can be seen in Table 2 that, A had 89.1 0.01 mg/100 g flavonoid, while B gave 63.5 0.01 mg/100 g. 263.1 0.01 mg/100 g and 249.7 0.02 mg/100 g alkaloids were obtained in A and B, respectively. Total phenols in A was 42.30 0.01 mg/100 g and 43.2 0.03 mg/100 g for B. Flour A gave 347.4 0.01 mg/100 g anthocyanins and B had 281.3 0.02 mg/100 g. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) inhibition (%) of 379.4 0.01 was found in A and 363.2 0.01 for B. Total phenolic content, 276.4 mg GAE/ 100 g and anthocyanins, 105.7 mg C3GE/100g were reported in literature [10]. The phenolic content ranges from 618.28 to 977.35 mg/100g [13]. More so, the values of these phytochemicals as reported are similar to those observed by Ossamulu et al. [21] as quoted in Table 2.
In view of these results, eggplant can help to reduce the nutrition-related disorders in Africa [13]. Eggplant is one of the most important vegetable crops known for its nutritive benefits due to the abundance of various bioactive compounds, which include proteins, vitamins, minerals, carbohydrates, phenolics, and dry matter content [22]. Eggplant produces secondary metabolites, including glycoalkaloids, antioxidant compounds, and vitamins, which appear to be the major source of its health benefits [22]. Also heat treatment used prior to consumption can increase the content and the biological action of antioxidant compounds of eggplants. This fact is due to the increase in phenolic compounds, such as caffeic and chlorogenic acids, which are known to be antioxidants that would promote greater removal of reactive oxygen species [3]. Wet cooking methods (boiling and pressure cooking) markedly increased fruit antioxidant capacity indicating that these preparation procedures improved antioxidant extractability [23].
Physiochemical properties
Also, the physiochemical properties are expressed in Table 3.
Table 3: Physicochemical Properties.
Key: Tit= titratable acidity. Values are mean ± SD of triplicates records
Values of 4.16 (in A) and 4.17 (for B) were detected as the pH; while titratable acidity indicated 0.44 0.01 g/L and 0.23 0.22 g/L for A and B, respectively. Conductivities observed were 515.3 0.58 µs/cm (A) and 512.3 1.53 µs/cm (B). These physicochemical parameters were comparable to as reported by Ossamulu et al. [21]; see the Table 3. The pH specifies the ability of a microorganism to grow in a specific food while the titratable acidity is a gives the impact of acid content on the flavor of food. On the other hand, colour is the first notable characteristics of food and often predetermines our expectations.
Functional properties
The functional properties are as given Table 4.
Table 4: Functional Properties.
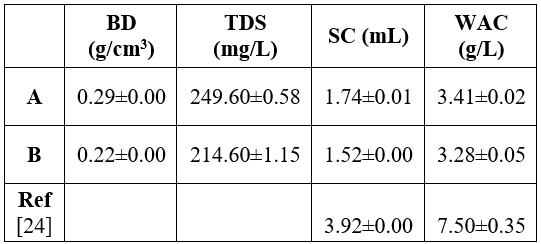
Key: TDS=total dissolved solids, WAC= water adsorption capacity, BD =bulk density, SC swelling capacity. Values are mean ± SD of triplicates records
Furthermore, A had water absorption capacity (WAC) of 3.41 0.02%, while B gave 3.28 0.65%. Swelling capacity of A and B were 1.74 0.01 mL and 1.52 0.00 mL, respectively. Meanwhile, the total dissolved solids (TDS) found were 249.6 0.58 mg/L (for A) and 214.6 1.15 mg/L (for B). For bulk density, A gave 0.29 0.00 g/cm3 and 0.22 0.00 g/cm3 was obtained for B. The swelling capacity of flours is a function of size of particles, types of variety and types of processing methods or unit operations [18]. The high bulk density of flour entails their suitability for use in food preparations. Nevertheless, low bulk density is also advantage in the formulation of complementary foods [18]. High WAC of composite flours implies that the flours can be used in formulation of some foods such as sausage, dough processed cheese and bakery products [18]. Eggplant is an agronomically and economically important plant member of Solanaceae family with a significant foundation source of various vital pharmaceuticals and nutraceuticals compounds [9][3]. It has been demonstrated that eggplants are good sources of essential nutrients, considering protein, fiber, ash, macro-elements, phenolics and source of antioxidants that make them therapeutically beneficial [13].
Mineral composition
The results of the mineral composition are given in Table 5.
Table 5: Mineral Composition.
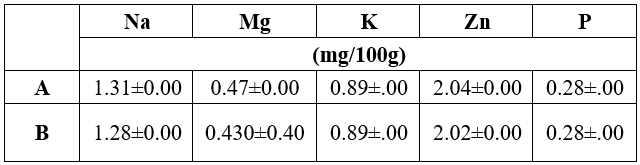
Of the minerals, Na, K, P, Zn, and Mg; Zn was the most present with levels of 2.04 0.00 mg/100 g in A and 2.02 0.00 mg/100 g for B; whereas, P recorded least amount. From literature we have seen that mineral content of eggplant showed a high concentration of minerals such as potassium (152.15 mg/ 100g), magnesium (25.35 mg/ 100 g), calcium (31. 36 mg / 100 g), sodium (8.49 mg / 100 g), zinc (0.51 mg / 100 g) [22]. During school-age, a higher consumption of these micronutrients can be beneficial, as it is associated to health growing and appropriate physical, cognitive and emotional development of children [10]. Again, eggplant features high fibre and low lipid contents, in addition to good content of minerals, especially manganese, zinc, copper, phenolic compounds and saponins with important in vitro antioxidant capacity [3]. Eggplants are a rich source of magnesium, manganese, potassium, and copper that are important for health bones. Eggplant is also known an Fe chelator that is suggested particularly for pregnant females, lactating mothers and teenagers’ females specifically. The Fe in eggplant has the ability to pact with pre-menstrual syndrome, amenorrhea, and antenatal anemia [9]. The addition of fruit juice powders into formulation used for snacks preparation is a promising approach towards the improvement of the functionally of ready-to-eat food products [25].
Conclusion
In this work it can be seen that drying temperature did not show any significant effect on the crude fibre of eggplant and the level of ash and protein. Moisture content of the flour is within the acceptable standard. Eggplant is a seasonal fruit, processing it into flour reduces postharvest losses. Hence eggplant flour could be developed and used as a functional ingredient in the food industry.
Declaration: The authors declare no conflict of interest.
Acknowledgements: The academic and technical staff of the Chemistry Department, Benue State University Makurdi, Nigeria are acknowledged for shaping this research technically.
References
- del Río-Celestino M and Font R. “The Health Benefits of Fruits and Vegetables,” Foods, 2020; 9(369): pp. 1–4.
- Rodriguez-Jimenez JR, Amaya-Guerra CA, Baez-Gonzalez JG, Aguilera-Gonzalez C, Urias-Orona V, Nino-Medina G. “Physicochemical, Functional, and Nutraceutical Properties of Eggplant Flours Obtained by Different Drying Methods,” Molecules, 2018; 23(3210): pp. 1–13.
- Scorsatto M, Rosa G, Luiz RR, da R P Mulder A, Teodoro AJ, aucia M M de Oliveira G. “Effect of Eggplant Flour (Solanum melongena L.) associated with hypoenergetic diet on antioxidant status in overweight women - a randomised clinical trial,” J. Food Sci. Technol., 2019; pp. 1–8.
- Philip TT, Asemave K, Obochi G. “Comparative Assessment of Phytochemicals in Four (4) Varieties of Ananas Comosus (l.) Merr Peels,” Chem. Biochem. Res., 2020; pp. 1–10.
- Asemave K, Tarhemba TP, Obochi GO. “Evaluation of Some Nutritional Profile of Pineapple (Ananas comosus (L.) merr) Rinds,” J. Green Herb. Chem., 2019; 9(1): pp. 026–037.
- Perfilova OV, Akishin DV, Vinnitskaya VF, Danilin SI, Olikainen OV. “Use of vegetable and fruit powder in the production technology of functional food snacks,” IOP Conf. Ser. Earth Environ. Sci., 2020; 548(082071): pp. 1–7.
- Cortbaoui PE, Ngadi MO. “Optimization of Postharvest Handling of Eggplant Using the Taguchi Technique,” Food Sci. Qual. Manag., 2018; 75: pp. 15–23.
- Silva GFP, et al. “Eggplant Fruit (Solanum melongena L.) and Bio-Residues as a Source of Nutrients, Bioactive Compounds, and Food Colorants, Using Innovative Food Technologies,” Sci., 2011; 11(151): pp. 1–23.
- Muhammad Yasir Naeem, Ugur S. “Nutritional Content and Health Benefits of Eggplant,” Turkish J. Agric. - Food Sci. Technol., 2019; 7(3): pp. 31–36.
- Soares JM, et al. “Eggplant Flour Addition in Cookie: Nutritional Enrichment Alternative for Children,” Foods, 2022; 11(1667): pp. 4–12.
- Nwanze NE, Nyorere O. “Mechanical Properties African Eggplant (Solanum aethiopicum L. Bello) Fruits, as Influenced by Maturation,” Direct Res. J. Eng. Inf. Technol., 2019; 6(2): pp. 14–17.
- Association of Official Analytical Chemist (AOAC). Official Methods of Analysis of International. 16th ed.; AOAC: Maryland, MD, USA, 1998.
- Combo AMM, Dakia PAA, Kobi OHJG, Angaman DM. “Investigating Eggplant Fruit (Solanum spp.) Cultivated in Côte d’Ivoire for Their Physicochemical and Antioxidant Characteristics,” Food Sci. Qual. Manag., 2020; 102: pp. 1–8.
- “AOAC, ‘Official methods of analysis’, VA: Association of Official Analytical Chemists, Arlington, Washington D.C.”, 1995.
- Anand SP, Seedar D, Velmurugan G. “Quantitative phytochemical analysis of some edible fruits from Boda and Kolli hills,” Pharmacogn. Phytochem., 2017; 6(5): pp. 2002–2005.
- Kumara P, Sunil K, Kumar BA. “Determination of DPPH Free Radical Scavenging Activity by RP-HPLC, Rapid Sensitive Method for the Screening of Berry Fruit Juice Freeze Dried Extract,” Prod. Chem. Res., 2018; 6(5): pp. 1–7.
- “AOAC (Association of Analytical Chemist, Official Methods of Analysis),” 7th ed., 2015; 1: pp. 112–120.
- Chandra S, Singh S, Kumari D. “Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits,” J Food Sci Technol., 2015; 52(6): pp. 3681–3688.
- Miranzadeh MB, Hassanzadeh M, Dehqan S. “Determination of total dissolved solid (TDS), nitrate and fluoride in 24 brands of Iranian bottled waters,” J. Phys. Sci., 2011; 6(22): pp. 5128–5132.
- Agoreyo BO, Obansa ES, Obanor E. “comparative nutritional and phytochemical analysis 0f two varieties of solanum melongena,” world J., 2012; 7(1): pp. 5–8.
- Ossamulu IF, Akanya HO, Edwin EF. “Evaluation of nutrient and phytochemical constituent of four eggplant flour,” Elixir food Sci., 2014; 73: pp. 26424–26428.
- Sharma M, Kaushik P. “Biochemical Composition of Eggplant Fruits: A Review,” Sci., 2021; 11(7078): pp. 1–13.
- Zaro MJ, Ortiz LC, Keunchkarian S, Chaves AR, Vicente AR, Concellon A. “Chlorogenic acid retention in white and purple eggplant after processing and cooking,” LWT - Food Sci. Technol., 2015; 64: pp. 802–808.
- Ndife J, Ojinaka MC. “Impact of blanching pretreatment on the quality characteristics of three varieties of oven dried eggplant,” 2019.
- Kita A, Nowak J, Michalska-Ciechanowska A. “The E ect of the Addition of Fruit Powders on the Quality of Snacks with Jerusalem Artichoke during Storage,” Sci., 2020; 10(5603): pp. 1–17.

