Adaptation of the Sars-Cov-2 Screening and Testing Strategy in Pregnant Women During the Second Wave of the Covid-19 Pandemic
Anja Štrbenc1, Tanja Premru Sršen2,3, Vesna Fabjan Vodušek2, Miha Lučovnik2,3, Marijana Vidmar Šimic2, Mirjam Druškovič2, Gorazd Kavšek2, Mario Poljak1 and Andreja Trojner Bregar2,3,*
1Institute of Microbiology and Immunology, Faculty of Medicine, University of Ljubljana, Slovenia
2Department of Perinatology, Division of Obstetrics and Gynecology, University Medical Center Ljubljana, Slovenia
3Faculty of Medicine, University of Ljubljana, Slovenia
Received Date: 30/11/2022; Published Date: 23/12/2022
*Corresponding author: Andreja Trojner Bregar, MD, PhD, Department of Perinatology, Division of Obstetrics and Gynecology, University Medical Center Ljubljana, Ljubljana, Slovenia and Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
Abstract
Background: Approaches to SARS-CoV-2 screening and testing of pregnant women vary among different obstetrics units and are usually tailored to the current epidemiological situation. The dramatic increase in the SARS-CoV-2 incidence in the autumn of 2020 prompted our hospital to adapt its protocol for SARS-CoV-2 screening and testing of pregnant women at admission.
Methods: To assess the usefulness of the updated SARS-CoV-2 screen-and-test approach, we retrospectively reviewed the Ljubljana Maternity Hospital database and searched for pregnant women admitted to the hospital between 27 October 2020 and 28 February 2021. SARS-CoV-2 real-time reverse-transcription polymerase chain reaction (RT-PCR) and rapid antigen test (RAT) results were retrieved from the database.
Results: During the four months analyzed, 2,552 hospitalizations and 5,516 outpatient visits occurred at our hospital. Records for nasopharyngeal swab testing using SARS-CoV-2 RAT were available for 1,836 questionnaire screen-negative women admitted for delivery, planned procedure, or hospitalization, of which 26 (1.4%) tested RAT positive. Subsequent RT-PCR testing identified the presence of SARS-CoV-2 RNA in 20/26 (76.9%) and RAT false-positive results in 6/26 (23.1%) cases.
Conclusions: During the peak of the COVID-19 pandemic in Slovenia, the updated screen-and-test approach using RAT with subsequent RT-PCR confirmation identified 1.1% of screen-negative pregnant women to be infected with SARS-CoV-2 at admission. Our experience may help efficient adaptation of screen-and-test approaches in obstetric units facing a surge in SARS-CoV-2 cases.
Keywords: SARS-CoV-2; Screening; Testing; Pregnancy; COVID-19; Slovenia
Introduction
During the first COVID-19 wave in the spring of 2020, Slovenia’s efficient tackling of the outbreak resulted in one of the lowest rates of infections and deaths per capita [1]. Unfortunately, the situation took a drastic turn in the autumn of 2020, forcing the Slovenian government to re-declare an epidemic on 19 October 2020. Strict COVID-19 measures were re-imposed, including the prohibition of assembly and movement between different municipalities, a 9 pm to 6 am curfew, a ban on sales of non-essential items, and closures of educational institutions. Despite stringent measures, rates of new COVID-19 cases exponentially increased and eventually propelled Slovenia to the top of the list of countries with the highest mortality per capita, currently listed as a country with the twelfth highest COVID-19-related cumulative mortality globally (207.76 deaths per 100,000 population) [2].
Whereas approximately 0.7% of the population had had a laboratory-confirmed SARS-CoV-2 infection at the beginning of the second wave, by the end of April 2021, the proportion rose to 11.3%. A change in COVID-19 demographics was also noted during the second wave – while residents and staff in nursing homes were most severely affected in spring 2020, the majority of new COVID-19 cases during the second wave consisted of people between the ages of 25 and 64 [2].
Hence, due to the high circulation of the virus within the younger, active populations, pregnant women were also more likely to have been exposed to SARS-CoV-2. In accordance, obstetric units had to promptly adapt their screen-and-test protocols for pregnant women before delivery.
Herein we describe and evaluate the transition from the screen-and-test approach implemented during the first COVID-19 wave (low incidence of SARS-CoV-2) [3] to the one implemented during the second COVID-19 wave (high incidence of SARS-CoV-2).
Methods
We retrospectively reviewed the Ljubljana Maternity Hospital medical database and searched for pregnant women, admitted to the Department of Perinatology, University Medical Centre Ljubljana, Slovenia between 27 October 2020 (one week after the SARS-CoV-2 epidemic was declared in Slovenia for the second time) and 28 February 2021 (beginning of the plateau with significantly lower rates of new COVID-19 cases, deaths, and hospitalizations). SARS-CoV-2 rapid antigen test (RAT) and real-time reverse-transcription polymerase chain reaction (RT-PCR) results were retrieved for pregnant women admitted for delivery, planned procedures, or hospitalization.
The screen-and-test approach used during the second wave is presented in Figure 1. As a part of the screening procedure at admission, pregnant women were asked to complete a questionnaire, described in detail in a previous report [3]. Briefly, pregnant women were asked about recent contacts with SARS-CoV-2-positive individuals, history of traveling, and COVID-19-compatible signs and symptoms during the two weeks prior to admission. Women with a low probability for SARS-CoV-2 infection (questionnaire screen-negative) were tested using Sofia SARS Antigen Fluorescent Immunoassay (FIA) (Quidel Corporation, San Diego, CA, USA) [4], whereas those with a documented SARS-CoV-2 infection in last 3 months were excluded from testing unless symptomatic. The Sofia SARS Antigen FIA is a rapid, instrument-based antigen test that received Food and Drug Administration (FDA) Emergency Use Authorization on 8 May 2020. The test enables qualitative detection of the SARS-CoV-2 nucleocapsid protein in nasal or nasopharyngeal swab samples.

Figure 1: The protocol of the screen-and-test approach is according to the type of patient evaluation. The part of the protocol that was analyzed in this study is delineated with a rectangle. At admission, pregnant women were screened using a questionnaire. Screen-positive women underwent RT-PCR testing, whereas RAT was used in screen-negative women, followed by subsequent RT-PCR confirmation of RAT-positive results. RT-PCR-positive women were managed at an isolation ward and/or isolated postpartum department. RAT=rapid antigen testing, RT-PCR=real-time reverse-transcription polymerase chain reaction, pos=positive, neg=negative.
All questionnaire screen-positive pregnant women (e.g., symptomatic, positive history for travel or recent contact with a SARS-CoV-2-positive individual) and all women with a positive Sofia SARS Antigen FIA test results were subsequently tested using either of the three RT-PCR-based methods: LightMix Modular SARS and Wuhan CoV E-gene kit (TIB Molbiol, Berlin, Germany) [5], Cobas 6800 SARS-CoV-2 Test (Roche Molecular Systems, Branchburg, NJ, USA) [6], or Alinity m SARS-CoV-2 Assay (Abbott Molecular, Des Plaines, IL, USA) (7), using the same internally validated cut-off value for a positive result. Questionnaire screen-positive and Sofia SARS Antigen FIA-positive pregnant women were accommodated in the isolation room or SARS-CoV-2 delivery ward until RT-PCR test results were available.
Pregnant women presenting to the outpatient department were only screened for COVID-19 using a questionnaire, whereas RT-PCR testing was performed only in those with COVID-19-compatible signs and/or symptoms. In this population, testing with the Sofia SARS Antigen FIA was not performed.
Finally, we compared the testing outcome during the first [3] and second epidemic periods. Patient confidentiality was ensured throughout the study and the data obtained were analyzed anonymously.
Results
During the period analyzed, there was a total of 2,552 hospitalizations and 5,516 outpatient visits at our Maternity hospital. As shown in Figure 1, screening with a questionnaire was performed in 2,414 hospitalized women, 578 (23.9%) were questionnaire screen-positive and 1,836 (76.1%) questionnaire screen-negative. Records for nasopharyngeal swab testing using SARS-CoV-2 RAT were available for 1,836 questionnaire-screen-negative women scheduled for hospitalization or with signs of the beginning of delivery. Of these, 26 (1.4%) tested positive using RAT and were subsequently tested for the presence of SARS-CoV-2 RNA. The presence of SARS-CoV-2 RNA was detected in 20/26 (76.9%) RAT-positive women, and RAT false-positive results were identified in 6/26 (23.1%) of pregnant women.
In contrast to the first epidemic period when none of the 202 tested pregnant women had been found SARS-CoV-2 infected, 20/1,836 (1.1%) of questionnaire screen-negative were considered to be infected with SARS-CoV-2 at admission (Figure 2).
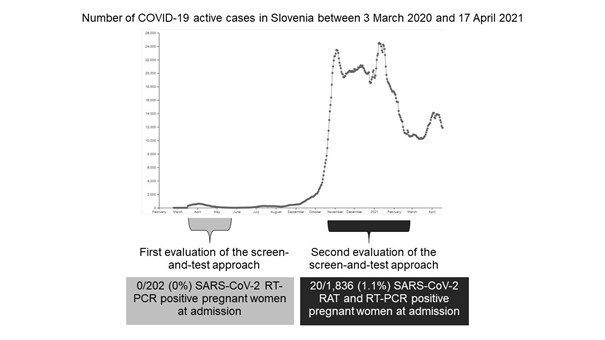
Figure 2: Comparison of testing outcomes during the first [3] and second screen-and-test evaluation period concerning the daily number of active COVID-19 cases in Slovenia between 3 March 2020 and 17 April 2021 (Internet Source: covid-19.sledilnik.org). RAT=rapid antigen testing, RT-PCR=real-time reverse-transcription polymerase chain reaction.
Discussion
The guidelines for the management of pregnant women admitted for delivery have regularly been updated concerning the evolution of the COVID-19 pandemic from the first to second and following waves [8]. Initially, screening of pregnant women admitted for hospitalization or delivery in Slovenia focused on travel history and the presence of COVID-19-compatible symptoms. Nevertheless, it soon became clear that, in addition to a screening questionnaire, determining the SARS-CoV-2 status using RT-PCR and/or RAT is necessary to apply isolation measures, enable rational use of Personal Protective Equipment (PPE), and prevent transmission of the virus [8]. Testing of all pregnant women admitted for a planned procedure or hospitalization using RT-PCR was possible during the first wave due to a relatively low burden of SARS-CoV-2 in the Slovenian population [3]. However, skyrocketing numbers of new SARS-CoV-2 cases and escalating demands for SARS-CoV-2 testing at the beginning of the second wave considerably challenged the timely availability of RT-PCR testing results in obstetric units. In addition, scaled-up RT-PCR testing at a national level somewhat prolonged the time-to-result, and limited supplies of testing reagents as well as a lack of highly trained laboratory personnel hindered universal RT-PCR testing of all pregnant women admitted to our hospital. Because of a much higher likelihood of being infected with SARS-CoV-2 compared to the first wave, the hospital’s screen-and-test approach had to be adapted accordingly during the second wave. Thus, RAT with Sofia SARS Antigen FIA was implemented on 27 October 2020 to enable rapid triage of pregnant women admitted for a planned procedure or hospitalization. Analyzer-based Sofia SARS Antigen FIA provides automated results within 15 minutes with 96.7% positive and 100% negative percent agreement with RT-PCR, according to the manufacturer data [4]. However, in real-life settings, the sensitivity, and specificity of the antigen test may be considerably lower and largely depend on the pre-test probability (e.g., asymptomatic vs. symptomatic; time since exposure) [9]. Despite the arguably lower analytical sensitivity and specificity of RAT compared to RT-PCR, RAT still allows rapid identification of infectious individuals and the potential earlier breaking of transmission chains [9]. Before the introduction of RAT in our Maternity Hospital, there were two instances of SARS-CoV-2 positive pregnant women being brought into the “white zone” (e.g., COVID-19 free zone) of the labour ward. One woman was asymptomatic, while the other denied any symptoms in the screening questionnaire she had filled out upon admission but became symptomatic later during the hospitalization.
In contrast to the first wave, screening and testing of all women admitted for delivery also became mandatory during the second wave. For this purpose, rapid antigen testing with Sofia SARS Antigen FIA was available 24/7. Namely, in addition to the symptomatic women, it is crucial to identify with regular testing also SARS-CoV-2 infected but asymptomatic women before they enter the delivery rooms allocated for COVID-19-negative patients. Potential intrusions into the “white zone“ are especially problematic during the birth process because of the potential risk for horizontal transmission to other mothers and new-borns within multiple-bed rooms. If RAT performed before delivery is negative, it is relatively safe for the staff to be in contact with a woman in labour for at least some time (possibly up to 24 hours), although testing should be immediately repeated if symptoms develop after initial testing or in case of confirmed SARS-CoV-2 infection among close contacts. In this study, only 20/26 (76.9%) of asymptomatic pregnant women that were RAT-positive were subsequently confirmed to be RT-PCR-positive. Hence, we believe that confirmation of a positive RAT result in asymptomatic pregnant women with RT-PCR is warranted. Our previous screen-and-test study performed during the first COVID-19 wave did not support universal SARS-CoV-2 RT-PCR testing since we had not identified a single new SARS-CoV-2-positive case among asymptomatic and even symptomatic pregnant women scheduled for a planned procedure at our institution [3]. In contrast, the current study clearly identified the benefit of universal screening and testing in settings with high SARS-CoV-2 burden. Because previous reports showed high rates (up to 89%) of asymptomatic SARS-CoV-2 infections among pregnant women [10,11], universal laboratory testing may provide a more accurate estimation of the prevalence of the disease in this population than screening for the presence of COVID-19 compatible symptoms using questionnaire only [12]. In addition, identifying SARS-CoV-2-positive women before delivery has several important clinical implications, including triage, allocation of appropriate resources (e.g., delivery ward, operating theatre) and PPE, transfer between hospitals, postpartum rooming-in, and neonatal care [13]. Universal SARS-CoV-2 testing of pregnant women should be preferably performed using clinically validated RT-PCR-based assays. However, despite its inferior analytical performance, testing with RAT may be an efficient tool for determining the SARS-CoV-2 status of pregnant women, providing that universal RT-PCR testing is unattainable. If rapid antigen testing is considered, a choice of the appropriate test is crucial, since a recent comparative evaluation of 122 CE-marked SARS-CoV-2 RATs showed that the sensitivity of different RATs varied over a wide range with potentially serious clinical consequences [14].
Whereas only two women were actively infected with SARS-CoV-2 at the time of delivery during the initial 1.5-month surveillance period during the first COVID-19 wave in Slovenia [3], a total of 119 deliveries needed to be carried out in a separate COVID-19 labour ward during the second surveillance period.
For a few days in November 2020, all three SARS-CoV-2 delivery wardrooms were always occupied. In addition, all five beds in the intensive care unit were occupied for rooming-in SARS-CoV-2-positive mothers and their new-borns; however, despite the heavy workload of the medical and support staff, we were able to perform all work needed without opening additional wards.
Our study has several limitations. We analysed a relatively short period (four months) and included data from a single center only. However, our Maternity Hospital is the biggest tertiary perinatal center in Slovenia, with almost 1/3 of all deliveries in the country (around 5,500 deliveries per year), accepting also in-utero transfers up to 34 gestational weeks as well as otherwise high-risk pregnant women from all other 13 obstetrical units within our country. Because of the study design we were, unfortunately, not able to evaluate the proportion of SARS-CoV-2 infected pregnant women that were missed due to using a screening test with inferior sensitivity compared to RT-PCR. However, in our evaluation, none of the RAT-negative women became symptomatic in the immediate postpartum period or hospitalization, suggesting triage with RAT may provide sufficient protection in most cases.
Conclusion
The results of this study confirm previous observations that the benefit of universal SARS-CoV-2 testing depends on the current epidemiological situation in the region/country. Thus, obstetric units should closely monitor local SARS-CoV-2 epidemiological situation and anticipate modifications in the screen-and-test protocol if a surge of new SARS-CoV-2 infections is observed within the community. Although RT-PCR is considered the gold standard for universal SARS-CoV-2 testing, a combination of RAT with RT-PCR may be more time- and cost-effective.
Conflict of interest: The authors declare that no conflicts of interest exist.
Funding: None.
Ethical Approval: As our study was a quality improvement project it does not meet the definition of human subject research. Therefore, according to Slovenian legislation, a review by the institutional review/ethical board was not required to analyse the data.
References
- Maver Vodičar P, Oštrbenk Valenčak A, Zupan B, Avšič Županc T, Kurdija S, Korva M, et al. Low prevalence of active COVID-19 in Slovenia: a nationwide population study on a probability-based sample. Clin Microbiol Infect, 2020; 26: 1514-1519. doi: 10.1016/j.cmi.2020.07.013.
- Johns Hopkins University & Medicine, Coronavirus Resource Center. Mortality analyses, 2021.
- Šterbenc A, Premru Sršen T, Lučovnik M, Vidmar Šimic M, Steblovnik L, Fabjan Vodušek V, et al. Usefulness of COVID-19 screen-and-test approach in pregnant women: an experience from a country with low COVID-19 burden. J Perinat Med, 2020; 49: 269-273. doi: 10.1515/jpm-2020-0368.
- Sofia SARS Antigen Fia, 2021.
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill, 2020; 25: doi: 10.2807/1560-7917.ES.2020.25.3.2000045.
- Poljak M, Korva M, Knap Gašper N, Fujs Komloš K, Sagadin M, Uršič T, et al. Clinical evaluation of the cobas SARS-CoV-2 test and a diagnostic platform switch during 48 hours in the midst of the COVID-19 pandemic. J Clin Microbiol, 2020; 58: e00599-20. doi: 10.1128/JCM.00599-20.
- Kogoj R, Kmetič P, Oštrbenk Valenčak A, Fujs Komloš K, Seme K, Sagadin M, et al. Real-life head-to-head comparison of performance of two high-throughput automated assays for detection of SARS-CoV-2 RNA in nasopharyngeal swabs: the Alinity m SARS-CoV-2 and cobas 6800 SARS-CoV-2 assays. J Mol Diagn, 2021; 23: 920-928. doi: 10.1016/j.jmoldx.2021.05.003.
- Wilcox W, Bajaj K, Rossberg MC, Knight C, Wieland D, Malhotra Y. Lessons learnt in transitioning from universal screening to universal testing of pregnant patients for SARS-CoV-2 at the largest municipal health system in America. J Perinatol, 2021; 1–3. doi: 10.1038/s41372-020-00889-4.
- Pray IW, Ford L, Cole D, Lee C, Bigouette JP, Abedi GR, et al. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses - Wisconsin, September-October 2020. MMWR Morb Mortal Wkly Rep, 2021; 69: 1642-1647. doi: 10.15585/mmwr.mm695152a3.
- Sutton D, Fuchs K, D'Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med, 2020; 382: 2163-2164. doi: 10.1056/NEJMc2009316.
- Khalil A, Hill R, Ladhani S, Pattisson K, O'Brien P. Severe acute respiratory syndrome coronavirus 2 in pregnancy: symptomatic pregnant women are only the tip of the iceberg. Am J Obstet Gynecol, 2020; 223: 296-297. doi: 10.1016/j.ajog.2020.05.005.
- Díaz-Corvillón P, Mönckeberg M, Barros A, Illanes SE, Soldati A, Nien JK, et al. Routine screening for SARS CoV-2 in unselected pregnant women at delivery. PLoS One, 2020; 15: e0239887. doi: 10.1371/journal.pone.0239887.
- Vintzileos WS, Muscat J, Hoffmann E, John NS, Vertichio R, Vintzileos AM, et al. Screening all pregnant women admitted to labor and delivery for the virus responsible for coronavirus disease 2019. Am J Obstet Gynecol, 2020; 223: 284-286. doi: 10.1016/j.ajog.2020.04.024.
- Scheiblauer H, Filomena A, Nitsche A, Puyskens A, Corman VM, Drosten C, et al. Comparative sensitivity evaluation for 122 CE-marked SARS-CoV-2 antigen rapid tests. medRxiv, 2021; 21257016. doi: 10.1101/2021.05.11.21257016.

