Subtherapeutic Antiepileptic Drug Levels Due to Chronic, Delayed Diarrhea Related to Dimethyl Fumarate
Ajaz Sheikh1,*, Maria Rabbani1 and Nazima Bashir2
1Department of Neurology, University of Toledo College of Medicine, Toledo OH, USA
2Department of Community Medicine, Government Medical College Srinagar, J&K, India
Received Date: 17/08/2022; Published Date: 01/09/2022
*Corresponding author: Ajaz Sheikh, Department of Neurology, University of Toledo College of Medicine, ProMedica Neurosciences Center, 2130 West Central Avenue, Toledo OH, USA
Abstract
Background: Multiple sclerosis and epilepsy are not uncommonly comorbid and treatment of one condition may affect the status of the other.
Objective: We here report a case of persistently sub-therapeutic antiepileptic drug levels related to diarrhea due to concomitant treatment with Dimethyl Fumarate in patient with epilepsy and multiple sclerosis.
Methods: Single case description
Results and Conclusion: Subtherapeutic antiepileptic drug level due to diarrhoea secondary to Dimethyl Fumarate is a potential complication to be borne in mind in patients with epilepsy and multiple sclerosis treated with this agent.
Keywords: Multiple Sclerosis; Epilepsy; Dimethyl fumarate; Diarrhoea
Introduction
Chilaiditi syndrome is a phenomenon of radio-clinical description [1]. It is a rare syndrome characterised by segmental interposition of the colon between the liver and the diaphragm [1-3]. It was reported by a Greek radiologist in 1910: Demetrius CHILAIDITI [2]. Its incidence in the world population is between 0.025 and 0.28% [2-3]. Chilaiditi syndrome is predominantly male, with a sex ratio of 4, and it is the preserve of the elderly [3]. The syndrome may be symptomatic (Chilaiditi sign), incidental or accompanied by clinical signs (Chilaiditi syndrome) [1,3]. These signs can be both digestive and thoracic [2]. The pitfall of this syndrome is that it can be distinguished from pneumoperitoneum [1]. The management of this syndrome may be conservative or may require surgical intervention [2]. We report a case of Chidailiti syndrome discovered in an elderly subject in Togo, which had a favourable evolution under conservative treatment.
Case History
Our case is a 36-year-old right-handed man, with the severe cognitive impairment and right frontal lobe epilepsy related to relapsing remitting multiple sclerosis (MS), who follows us regularly in the epilepsy clinic. His seizures began at the age of 19 years, and were determined to be due to underlying MS. Seizure semiology is described as staring, followed by head version to left, followed by left arm tonic seizure, and finally generalized tonic-clonic activity. He has been on multiple antiepileptic drugs (AEDs) including Lacosamide (LCM), Phenytoin (PHT), Levetiracetam (LEV), Valproic acid (VPA) and Perampanel (PER) (doses & timelines in Table 1). Because of intractable seizures, he also had Vagus Nerve Stimulator (VNS) implanted in 2017. He was started on Dimethyl Fumarate (DMF) 240 mg twice daily as a disease modifying therapy for MS in 2015.
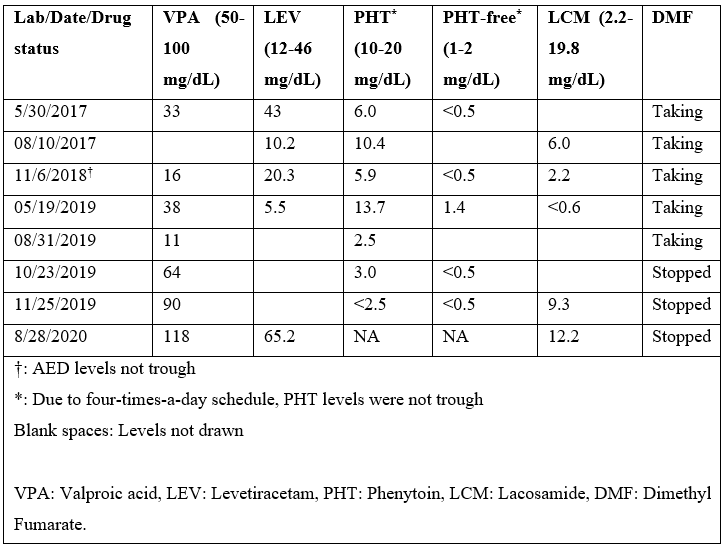
He started following our epilepsy clinic in 2016, and most of the medical records prior to that were not available. In 2017, he had 2 hospital admissions due to breakthrough seizures secondary to sub-therapeutic PHT and VPA levels (Table 2), despite excellent compliance to the medications. VPA dose was increased from 500mg to 1250mg bid, and he was seizure free for 8 months on four AEDs (Table 1). In January 2018, patient’s mother brought to our attention his diarrhoea and excreting LEV pills intact. The diarrhoea had apparently been going on for some time already. LEV was switched to liquid formulation. He was also referred to gastroenterology for evaluation for malabsorption, which returned unremarkable. He started having more frequent seizures around May of 2018, as the diarrhoea continued to get worse. All his AEDs were switched to liquid formulation, due to concern for malabsorption. After 2018, he was hospitalized three-times for breakthrough seizures, as he continued to have sub-therapeutic AED levels (Table 2). After ruling-out other potential causes for diarrhea, DMF was suspected to be the cause. DMF was switched to ocrelizumab in September 2019, resulting in prompt resolution of diarrhea. The subsequent AED levels (except for PHT) returned to therapeutic range. The PHT levels were consistently low since its initiation (some levels in table-2 were not trough) even prior to diarrhoea, and he was determined to be a rapid metabolizer. Afterwards, PHT was switched to PER 2 mg daily. Since stopping DMF, the AED levels have been consistently normal, and he has been seizure free.
Table 1: Chronology of AEDs Doses.
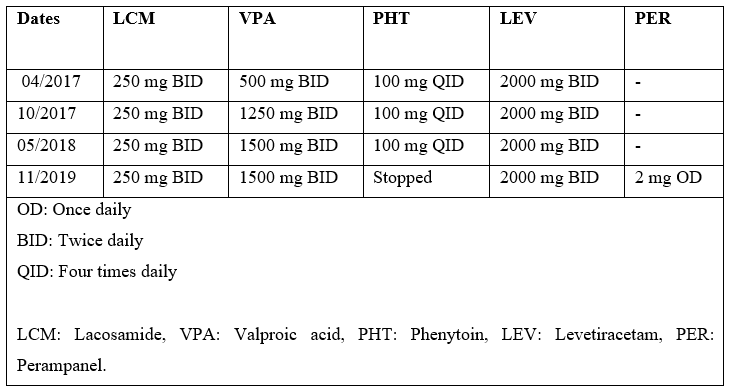
Table 2: Chronology of AED Levels in Relation to DMF Intake.
Discussion
Epilepsy and multiple sclerosis are both relatively common neurological disorders, and it’s not unusual for patients with MS to have seizures. According to one study, in patients with MS, cumulative incidence of epilepsy was 3.5% (2.2% for relapsing-remitting and 5.5% for progressive MS) [1].
The efficacy and safety of delayed-release DMF in relapsing-remitting MS was established based on two randomized, double-blind, placebo-controlled studies; DEFINE [2] and CONFIRM [3]. Gastrointestinal (GI) events are among the most common adverse effects (AE) related to DMF treatment. Based on a study, these events occurred most frequently in the first month, declining thereafter; most were rated as mild or moderate in severity (91%−96%), and resolved during the study (93%−96%) [4].
According to data from MANAGE, a multicenter, open-label, single-arm study, examining the GI events in patients with relapsing MS treated with DMF, although 88.4% patients reported a GI related adverse effect, only 1.7% patients experienced at least one serious GI adverse effect (including diarrhea, abdominal pain, nausea, salivary gland calculus, & vomiting), & 7.3% patients discontinued DMF due to GI side effect [5].
According to the DMF safety data in Phase III trials, with cumulative drug-exposure data of 2244 person-years [6], gastrointestinal AE, particularly nausea, vomiting, abdominal pain, and diarrhea, were more frequent in the DMF compared to placebo group (40% vs 31%, respectively) [2-4]. In most cases, these AE were mild or moderate in severity, and were seen most frequently in the first month of treatment, progressively decreasing afterwards [2,3].
In our patient, DMF was the most likely cause of diarrhea, since an extensive work up for chronic diarrhea was negative, and it resolved promptly and completely after stopping DMF. However, unlike the typical temporal characteristics, diarrhea in our patient was delayed, starting a few years after starting DMF, and persistent.
Although, classically, the GI side effects are transient and improve with time without the need to change the disease modifying therapy (DMT), our patient needed a switch in the DMT for resolution of diarrhoea. Among the recommendations from clinicians with experience using DMF, for avoiding GI adverse effects related to DMF, are administering DMF with protein and fat-rich foods (particularly for nausea and abdominal pain), slower titration to target dose (>7 days), temporary dose reduction, and use of symptomatic therapies (loperamide and diphenoxylate/atropine for diarrhoea) [7].
This case highlights the importance of periodically evaluating MS patients taking DMF for GI related adverse effects, particularly those with seizures who are on AEDs. If diarrhoea is present, a careful follow-up of AED levels may be necessary to rule out sub-therapeutic or fluctuant levels, which may lead to poor seizure control.
Brief disclosure: Ajaz Sheikh, Maria Rabbani and Nazima Bashir have no disclosures to make
Funding: None
References
- Burman J, Zelano J. Epilepsy in multiple sclerosis. A nationwide population-based register study. Neurology, 2017; 89: 2462-2468.
- Gold R, Kappos L, Arnold DL. DEFINE Study Investigators et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med, 2012; 367: 1098–1107.
- Fox RJ, Miller DH, Phillips JT. CONFIRM Study Investigators et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med, 2012; 367: 1087–1097
- Meltzer L, Selmaj K, Gold R, et al. Gastrointestinal tolerability events in relapsing-remitting multiple sclerosis patients treated with BG-12 (dimethyl fumarate) in DEFINE and CONFIRM. Neurology, 2013; 80(meeting abstracts 1): P01.164.
- Fox EJ, Vasquez A, Grainger W, et al. Gastrointestinal Tolerability of Delayed-Release Dimethyl Fumarate in a Multicenter, Open-Label Study of Patients with Relapsing Forms of Multiple Sclerosis (MANAGE). Int J MS Care, 2016; 18: 9-18.
- Tecfidera (prescribing information).
- Phillips JT, Erwin AA, Agrella S, et.al. Consensus Management of Gastrointestinal Events Associated with Delayed-Release Dimethyl Fumarate: A Delphi Study, Neurol Ther, 2015; 4: 137–146.

