Design and Development of Bilayer Tablets of Metformin Hydrochloride Sustained Release and Glimepiride Immediate Release for the Treatment of Type 2 Diabetes Mellitus
Abhishek Raj1,* and Amit Sharma2
1Department of Medicine, Jagannath University, India
2Dean (Medical, Paramedical & Allied Health Sciences) and HOD (Pharmacy), Jagannath University, India
Received Date: 27/07/2022; Published Date: 08/08/2022
*Corresponding author: Abhishek Raj, PhD Scholar, Department of Medicine, Jagannath University, Jaipur, Rajasthan, India
Abstract
The primary objective of this study was to formulate bilayer tablets of metformin hydrochloride (sustained release) and glimepiride (immediate release) in which the dose of metformin was 500 mg and the dose of glimepiride was 1 mg and 2 mg. The tablets were prepared by wet granulation. In this study, glimepiride layer was formulated in the form of immediate release as an initial dose and metformin layer was in the form of sustained release to act as maintenance dose. The sustained release layer of metformin was prepared by using HPMC K100 M polymer. The prepared tablets were evaluated for various physicochemical parameters such as drug-excipient interaction by FTIR, flow properties, hardness, weight variation, thickness, friability, disintegration time for glimepiride layer, in vitro dissolution studies, assay and uniformity of content. The formulated tablets were found to be similar in drug release profile to that of market sample. These tablets were also subjected for real time and accelerated stability studies as per ICH guidelines. These tablets were found to be stable even after 6 months of stability study as all the parameters were within limit as per the specifications.
Keywords: Diabetes; Metformin Hydrochloride; Glimepiride; Bilayer tablets; Sustained release; Antidiabetic; HPMC K100 M
Introduction
Bilayer tablet is suitable for sequential release of two drugs in combination. Here, the glimepiride layer is formulated to obtain immediate release of drug, with the aim of reaching a high serum concentration in a short period of time. The metformin layer is in the form of sustained release which is designed to maintain an effective plasma level for a prolonged period of time. The pharmacokinetic advantage relies on the fact that drug release from fast releasing layer leads to a sudden rise in blood concentration. However, the blood level is maintained at steady state as the drug is released from the matrix of the sustained release layer. The bilayer tablets of metformin and glimepiride is used, with diet and physical exercise to treat type 2 diabetes mellitus. This combination product is used in case the use of glimepiride or metformin alone does not control blood glucose level.
Materials and Methods
The following raw materials were used for preparing bilayer tablets of metformin hydrochloride and glimepiride by wet granulation technique in which metformin was in layer 1 and glimepiride was in layer 2. This was a unique formulation in which metformin was in sustained release form and glimepiride was in immediate release form. Two different optimized batches were prepared in which the strength of metformin was 500 mg in each case whereas the strength of glimepiride was 1 mg and 2 mg respectively.
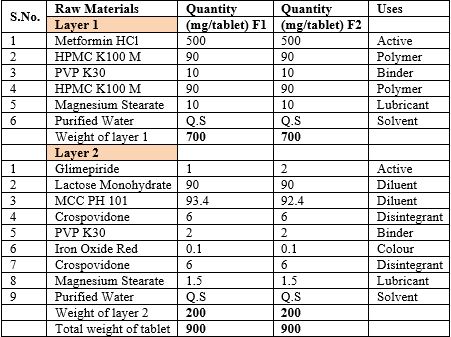
Table 1: Raw materials, specification, quantity and their uses in tablets.
Sieving, Mixing, Granulation and Lubrication of Layer 1
Metformin Hydrochloride and HPMC K 100 M were sieved through 40 mesh (#40). Metformin Hydrochloride and first part of HPMC K 100 M were mixed at slow speed in RMG for 5 minutes. PVP K30 was dissolved in water and used as a granulating solution. Granulation was done in RMG with the help of granulating solution until desired granulation was achieved. The wet granules were passed through 16 mesh (#16) and dried in FBD with inlet temperature of 550C until the moisture content was below 3%. The dried granules were passed through 20 mesh (#20) and second part of HPMC K 100 M was mixed with it in RMG for 5 minutes. Magnesium Stearate was sieved through 60 mesh (#60) and used for lubrication. Lubrication was done in RMG for 45 seconds.Sieving, Mixing, Granulation and Lubrication of Layer 2Glimepiride was geometrically diluted with lactose and sieved through 40 mesh (#40). MCC PH 101 and first part of Crospovidone were also sieved through 40 mesh (#40). All the ingredients were mixed in RMG at slow speed for 5 minutes. PVP K30 and Iron Oxide Red were dissolved in water and used as granulating solution. Granulation was done in RMG with the help of granulating solution until desired granulation was achieved. The wet granules were passed through 16 mesh (#16) and dried in FBD with inlet temperature of 550C until the moisture content was below 3%. The dried granules were passed through 20 mesh (#20) and second part of Crospovidone was mixed with it in RMG for 5 minutes. Magnesium Stearate was sieved through 60 mesh (#60) and used for lubrication. Lubrication was done in RMG for 45 seconds.The moisture content of the lubricated granules was observed in Denever digital moisture analyzer at 105°C. The angle of repose (Ɵ) was also performed for the lubricated granules and was found to be less than 30° and hence it reveals good flow property. The pre-compression parameters of the bulk lubricated powder were performed using Electrolab Tapped Density Tester USP.
Table 2: Moisture content and Angle of Repose.
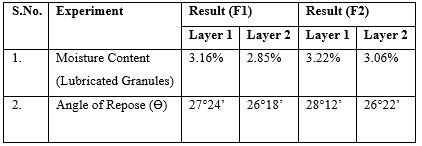
Table 3: Pre-compression parameters of bulk lubricated powder.

Compression
After performing the pre-compression parameters, the lubricated powder was subjected for compression using Karnavati 9 station double rotary tablet punching machine. The punching tools used were oblong, biconvex, 19.5 x 9.0 mm plain on both sides. The machine RPM was 15. The average punch weight of the tablets was 900mg in which the average weight of layer 1 was maintained as 700 mg and average weight of layer 2 was maintained as 200 mg. The hardness, thickness, weight variation and friability of the punched tablets were maintained in the desired range.
Hardness
Tablets require a certain amount of strength or hardness to withstand mechanical shocks of handling in manufacturing, packing and shipping. The hardness of the tablets was maintained between 90 to 120 Newton. Hardness was measured using digital Campbell Electronics tablet hardness tester.
Thickness
The thickness of tablets should be maintained to overcome packing problems. The thickness of punched tablets was maintained in the range of 6.2 to 6.6 mm with the average thickness of 6.3 mm. Thickness was measured using Dial Vernier Caliper.Weight Variation
The average percentage weight variation was within ±7.5% i.e. in the pharmacopoeia limit.
Friability
The friability test was performed using Electrolab Tablet Friability Tester. This is important to know the mechanical strength of tablets while handling. The friability of the punched tablets was found to be 0.19% (F1) and 0.22% (F2).
Disintegration
Disintegration time of the punched tablets (layer 2 consisting of Glimepiride) was done using Veego Disintegration Test Apparatus. One tablet was placed in each of the 6 tubes of the basket and disintegration was carried out using water maintained at 37°±2°C. The time taken for complete disintegration of the Glimepiride layer of tablets with no palpable mass remaining in the apparatus was measured and recorded. The minimum disintegration time was 50 seconds and the maximum disintegration time was 1 minute 45 seconds for these tablets. The average disintegration time was 1 minute 25 seconds (F1) and 1 minute 45 seconds (F2).
Table 4: Summary of Post-compression parameters.


Graph 1: Dissolution of Glimepiride Layer (F1 and F2).
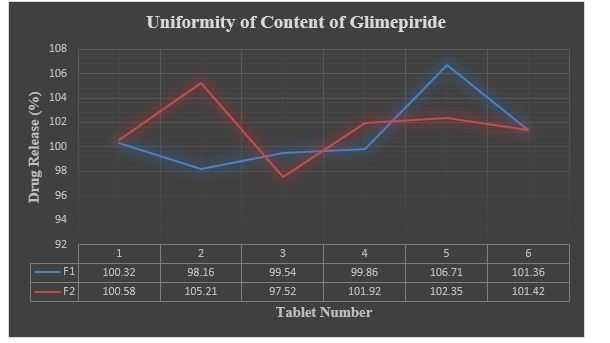
Graph 2: Uniformity of Content of Glimepiride (F1 & F2).
Method of Analysis
Identification of Glimepiride:
In the assay, the principal peak in the chromatogram obtained with the sample solution corresponds to the peak in the chromatogram obtained with the standard Glimepiride RS solution.
Identification of Metformin HCl:
The light absorption spectrum of the sample in the range from 210 nm to 310 nm should correspond to be with the absorption spectrum of working standard in the assay.
Dissolution of Glimepiride:
Dissolution parameters
Medium: 900 ml phosphate buffer pH 7.8
Apparatus: Paddle
Temperature: 37±0.5°C
Revolution: 100 RPM
Time: 45 minutes
Preparation of 1000 ml Phosphate buffer pH 7.8
Dissolve 0.58 gram of monobasic potassium phosphate and 8.86 gram of anhydrous dibasic sodium phosphate in 1000 ml water. Adjust the pH to 7.8 with dilute phosphoric acid or 1 M sodium hydroxide.
Standard Preparation:
Weigh accurately 25 mg Glimepiride WS in 200 ml volumetric flask and dissolve it with 100 ml of 90% acetonitrile by sonicating for 5 minutes. Allow to cool at room temperature & make up the volume with same. Dilute 2 ml of this solution to 200 ml with dissolution medium. Further dilute 10 ml of this solution to 25 ml with 50% methanol & filter through 0.2 micron filter paper.
Sample Preparation:
Place 1 tablet in each dissolution jar and run the apparatus as per above dissolution parameters. Collect 15 ml of sample from each vessel at specified time interval and filter. Dilute 10 ml of this filtered solution to 25 ml with 50% methanol & filter through 0.2 micron-filter paper.
Procedure:
Proceed as prescribed in the assay method using 50µl injection volume and calculate the dissolution in percent of each tablet with respect to claim amount using the formula.
Calculation:
Area of Sample X Wt. of Standard (g) X 9 x 100-Water % X Potency % WS X Average wt. (mg/tab)
Area of Standard 0.001 200 100
Result: Glimepiride %
Uniformity of content
Glmepiride:
Sample preparation: weigh 10 tablets individually and place one tablet in each of 10 in 100 ml volumetric flask, add about 70 ml of 90% acetonitrile. Shake well and sonicate for about 20 minutes. Cool at room temperature and make up the volume to mark with same solvent. Centrifuge if necessary and filter the solution through 0.2 micron filter paper.
Standard Preparation: Weigh about 25 mg of glimepiride working standard in 100 ml volumetric flask, add about 70 ml of 90% acetonitrile and sonicate for about 5 minutes. Cool to room temperature and make up the volume to mark with same solvent. Dilute 2ml to50 ml with same solvent and filter through 0.2 µm filter paper.
Procedure: Proceed as prescribed in assay method, using 10µl injection volume.
Calculation:
Area of Sample X Wt. of Standard (g) X 2 x 100-Water % X Potency % WS
Area of Standard Avg. Assay (g) 50 100
Result: Glimepiride %
Assay
Glimepiride:
Instrumentation and requirements
1. Chromatograph:
Pump Shimadzu XR gradient system with two pumps
Detector Shimadzu XR with semi micro flow cell
Column Octadecylsilane, 2.6 micron particle size, Dimension 150 x 4.6 mm (sunshell)
Injection Shimadzu XR Auto sampler
Column Oven Shimadzu XR
2. Chromatography Conditions:
Temperature 35°C
Detection 228nm
Run time 10 min
Flow rate 1.0ml/min
Injection Volume 10µl
3. Mobile Phase
Weigh accurately 250 g of NaH₂PO₄ and dissolve in 250 ml HPLC water, and adjust pH to 2.5 with Phosphoric acid (10%). Mix with 250 ml HPLC grade acetonitrile and sonicate for 10 minutes. Cool to room temperature and filter the solution through 0.2 µm filter paper using vacuum pressure.
Method of Analysis
Standard Preparation:
1. Glimepiride WS:
Weigh accurately 25mg of Glimepiride working standard and transfer in 100 ml volumetric flask. Add about 70 ml of 90% acetonitrile and sonicate for 5 minutes, cool to room temperature, make up the volume to 100 ml with same solvent. Further dilute 2ml of this solution to 50 ml with same solvent and filter through 0.2μm filter paper.
2. Sample Preparation:
Weigh 20 tablets and find the average weight per tablet. Crush the 20 tablets and mix homogeneously with the help of mortar and pestle. Weigh accurately tablet powder equivalent to 1mg Glimepiride and transfer in 100 ml volumetric flask. Add 70 ml of 90% acetonitrile and sonicate for about 20 minutes. Allow to cool at room temperature and make up the volume to mark with same solvent. Centrifuge the solution for 5 minutes and filter through 0.2µm filter paper.
3.Procedure:
Separately inject 10μl of standard preparation and the sample preparation into the injection valve and note the chromatographic area for quantification.
System suitability:
1.Glimepiride:
In the chromatogram obtained from the standard preparation, the column efficiency determined from the major peak is not less than 1000 theoretical plate, the tailing factor is not more than 2.0 and the relative standard deviation of replicate injections is not more than 2.0%.
Calculation:
Glimepiride:
Area of Sample X Wt. of Standard (g) X 2 x 100-Water % X Potency % WS X Average weight (mg/tab)
Area of Standard Wt. of Sample (g) 50 100 100
Dissolution:
Metformin HCl
1 Dissolution Parameter:
2 Medium: 1000 ml Phosphate buffer pH 6.8
3 Apparatus: Paddle
4 Revolution: 100 RPM
5 Temperature: 37 ± 0.5°C
6 Time: 1 hour, 3 hours and 10 hours
Phosphate buffer pH 6.8:
Dissolve 6.8 g of monobasic potassium phosphate in 500 ml water. Add 112 ml 0.2N Sodium hydroxide solution and then dilute to 1000 ml with water. Adjust the pH to 6.8 with 0.2 N NaOH if necessary.
5.2.1 Standard Preparation:
Weigh accurately 50 mg of Metformin HCl WS in 250 ml volumetric flask. Dissolve it in about 200 ml of dissolution medium and make up the volume with same medium. Dilute 5 ml of this solution to 100 ml with the dissolution medium.
Procedure:
Place one tablet in each dissolution vessel with help of suitable sinker and run the apparatus as per above dissolution parameters. Withdraw 10 ml of sample from each dissolution vessel at specified time interval and filter. Replenish each vessel with 10 ml of dissolution media after each sampling. Measure the absorbance of the sample and standard solution in UV-Vis spectrophotometer at about 232 nm and calculate the result by comparison method.
Calculation:
Calculate the dissolution in percent of each tablet at required time interval with respect to claimed amount using the below formula.
First Hour:
Sample dilution: 2 ml to 50 ml with dissolution media
Abs of Sample X Wt. of Std (mg) X 5 x 1000 X 50 X 100-Water % X Potency % WS
Abs of Standard 250 100 Claim (mg) 2 100
Third Hour:
Sample dilution: 2 ml to 100 ml with dissolution media
{ Abs of Spl X Wt.of Std (mg) X 5 x 1000 X 100 X 100-Water % X Potency % WS + A} x 100 ÷ claim in mg
Abs of Std 250 100 100 2 100
Tenth Hour:
Sample dilution: 2 ml to 100 ml with dissolution media
{ Abs of Spl X Wt.of Std (mg) X 5 x 1000 X 100 X 100-Water % X Potency % WS +A+B} x 100 ÷ claim in mg
Abs of Std 250 100 100 2 100
Results: Metformin HCl %
Where,
A- Amount of Metformin HCl (mg) sample during first hour analysis
B-Amount of Metformin HCl (mg) sample during third hour analysis
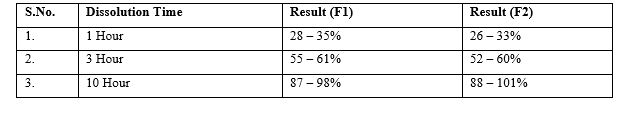
Table 5: Summary of Dissolution (Metformin Layer).
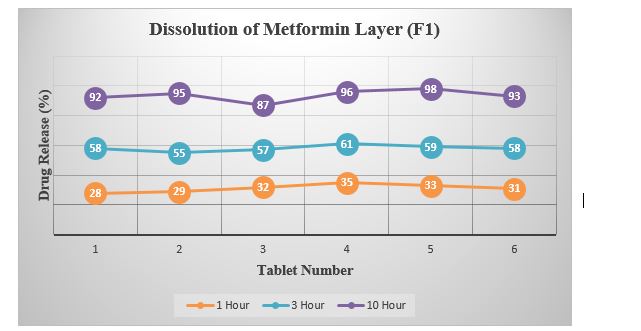
Graph 3: Dissolution of Metformin Layer (F1).
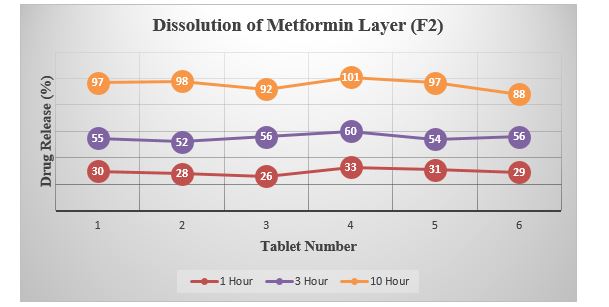
Graph 4: Dissolution of Metformin Layer (F2).
Assay:
1. Metformin HCl:
2. Instrumentation and requirements
3. Instrument: UV-Visible spectrometer
4. Diluents: Water
5. Conditions
Temperature: Ambient
Detection: 232 nm
6.Standard Preparation:
Weigh accurately about 50 mg of metformin HCl WS in 250 ml volumetric flask. Add 200 ml of diluent and shake for few minutes and sonicate for 25 minutes. Cool, make up the volume to mark with same diluent. Dilute 5 ml of this solution to 100 ml with same diluent.
7.Sample Preparation:
Weigh 20 tables and find the average weight per tablet. Crush 20 tablets and mix homogeneously with the help of mortar and pestle. Weigh accurately tablet powder equivalent to 50 mg of Metformin HCl and transfer in 250 ml volumetric flask. Add 200 ml of diluent; shake for few minutes and sonicate for 25 minutes for complete dissolution; cool the solution to room temperature ad make up the volume with same diluent. Sediment or centrifuge the solution at 2000 RPM for 10 minutes. Dilute 5 ml of this solution o 100 ml with same diluent.
8.Procedure:
Measure the absorbance of both standard and sample solution at maximum at about 232 nm and calculate the results by comparison.
9.Calculation:
Metformin HCl
Area of Sample X Wt. of Std (g) X 2 x 100-Water % X Potency % WS X Avg. wt. (mg/tab)
Area of Standard Wt. of Spl (g) 50 100 100
Result: Metformin HCl %
Packing: The primary packing of these tablets was done in clear PVDC blister. 10 tablets were packed in each blister.
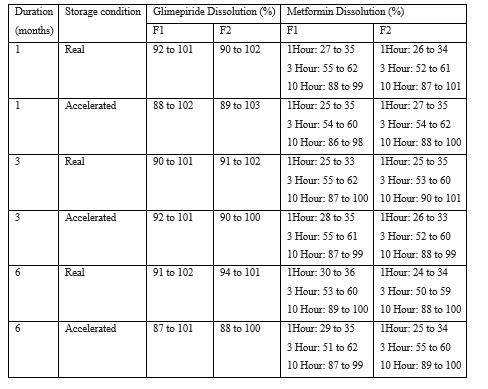
Stability Studies: After blister packing, these tablets were subjected for real time and accelerated stability studies as per ICH guidelines for 6 months. The real time stability was carried out by storing the tablets at 30°C ± 2°C and 75% ± 5% RH. The accelerated stability was carried out by storing the tablets at 40°C ± 2°C and 75% ± 5% RH in Thermolab Stability chambers.
Table 4.1: Summary of stability report.

Table 4.2: Summary of stability report.
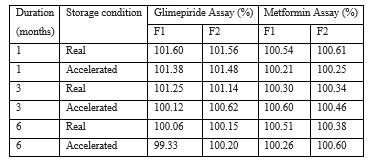
Table 4.3: Summary of stability report.
Conclusion
Bilayer tablets of Metformin Hydrochloride Sustained release and Glimepiride immediate release were successfully formulated by employing wet granulation method. HPMC K 100 M was used as sustained release polymer in Metformin Hydrochloride layer and crospovidone was used as superdisintegrant in Glimepiride layer. Pre-compression parameters such as bulk density, tapped density, Carr’s Index, Hausner’s ratio and angle of repose was done for the bulk lubricated granules and this revealed good flow and compressibility property of the bulk granules. Post-compression parameters such as hardness, thickness, weight variation, friability, disintegration (for glimepiride layer only), dissolution, assay and uniformity of content (for glimepiride) was performed on punched tablets and all the parameters was found to comply with the in-house specification. Real and accelerated stability study was carried out for a period of 6 months as per ICH guidelines and the formulation was examined for physical appearance, average weight, disintegration time, hardness, assay and dissolution. All the tests showed satisfactory results according to in house specifications.
References
- Lachman Leon, Liberman Herbert A, Kanig Joseph L. The Theory and Practice of Industrial Pharmacy. 3rd edition, Varghese publishing house; 1987: 296-303.
- Lee VH. and Robinson JP, Sustained and Controlled release drug delivery system, Marcel Dekker, New York, 1978: 7-11.
- Remington, The science and practice of pharmacy: Twentieth Edition; 903-914.
- Pharmaceutical dosage forms: Tablets, Revised and Expanded: Lieberman, Lachman and Schwartz, Vol – I, 2nd
- Jens T Cartensen. Drug Stability Principles and Practices; 1990; 394-399.
- Lachman Luberman, Kanig Lea, Febiger P. The theory and practice of Industrial Pharmacy. 3rd edition, 66 – 68.
- Siepmann J, Kranz H, Bodmeier R. HPMC Matrices for Controlled Drug Delivery: A New Model Combining Diffusion, Swelling and Dissolution Mechanisms of Predicting the Release Kinetics, Pharm Res, 1999; 16: 1748 – 1756.
- Viazquez MT, Perel-Marcos B, Gomez-Amoza JL, Martiner Pache Cho R, Souto C, Concheiro A. An Influence of Technological Variables on Release of Drugs from Hydrophilic Matrices, Drug Dev Ind Pharm, 1992; 18: 1355-1375.
- Alferd Goodman Gliman, Lee E Limbird, Joel G Hardman. The pharmacological basis of therapeutics, Mc Graw hill, 10th edition, 1701-1707.
- United States Pharmacopoeia (U.S.P.), 2018; 1 and 2.
- Indian Pharmacopoeia (I.P.), 2018; 1, 2 and 3.
- Handbook of Pharmaceutical Excipients 6th edition, 2009.
- Gilbert S Banker, Christopher T Rhodes. Modern Pharmaceutics, 4th edition, 2002; 504.
- Richard D.Howland, Mary J Myek, Lippincotts illustrated reviews pharmacology, 3rd edition, 287-293.
- Defronzo RA. Pharmacologic Therapy for Type 2 Diabetes Mellitus, Ann.Intern. Med, 1999; 131(4): 281-303.
- James Swarbrick. Encyclopedia of Pharmaceutical Technology, 3rd edition, 2007; 2: 1082.
- International Conference on Harmonization (ICH), Harmonized Tripartite guideline for stability testing of new drug substances and products, Q1A (R2), 2003.
- Abhishek Raj. Formulation and in-vitro evaluation of Voglibose dispersible tablets. European journal of biomedical and pharmaceutical sciences, 2016; 3(2); 226-230.
- Abhishek Raj. Formulation and in-vitro evaluation of fast disintegrating Levocetirizine Dihydrochloride tablets, Journal of Atoms and Molecules 2015; 5(2); 910-915.
- Ingle PV, Talele GS. Rationale behind the combination of sulfonylurea and metformin in diabetes mellitus. International Journal Pharmaceutical Science and research. 2010; 1:1-5.
- Ding X, Robinson JR. Extended release and targeted drug delivery system. In Remington: The Science and Practice of Pharmacy. 20th edition, Gennaro; A. R., Ed. Lippincott Williams and Wilkins, 2000; 1: 939.
- Walker R, Edwards C. Clinical pharmacy and therapeutics, Churchill Livingstone., 2003; 3rd edition: 657 - 660.
- Podczeck F, Drake KR, Neton JM, Harian I. The strength of bi-layered tablet. European Journal of Pharmaceutical Sciences. 2008; 1-14.

