Assessment of Angiogenesis in Ameloblastoma: A Comparison Base on Patient Age
Hafiza Shahzadi Maryam*, Shamsul Hadi, Fazal Hanan, Humaira Saif and Alamgir
Department of Pathology, Peshawar Medica College, Pakistan
Received Date: 28/06/2022; Published Date: 08/07/2022
*Corresponding author: Hafiza Shahzadi Maryam, Department of Pathology, Peshawar Medica College, Peshawar, Pakistan
Abstract
Ameloblastoma is the most common odontogenic tumor derive from odontogenic epithelial cells. It’s a slow-growing tumor with an aggressive biological behavior including invasion of the surrounding bone and with high recurrence rate. Ameloblas- toma is a rare kind of cancer in persons in their fourth and fifth decades. It is the most often occurring type of cancer in this age group. The majority of patients with ameloblastoma are between the ages of 30 and 60. Mean Vascular Density (MVD) is a quantitative measure of angiogenesis determined using various markers, including CD 31, 34, and 105. Microscopic analysis of H&E slides was performed to confirm the diagnosis and determine which slides would be used for IHC (immunohistochemical) staining. The letter was performed using a Mouse Anti-Human CD 34 Monoclonal Antibody. The MVD was calculated as the mean of three randomly chosen locations for each micro vessel. Objects that start from a single blood vessel were counted if they are entirely detached from it. Musculature-lined blood arteries were removed. Statistical significance was determined by the probability value (p0.05). The study discovered that the second and third decades of life are the most common age groups for developing ameloblastoma, with an average age of 36.13+ 16.0 years. Ameloblastoma was most common in people in their second and third decades of life, respectively. younger patients are more likely to develop a plexiform form of ameloblastoma than older patients, and that elderly patients are more likely to develop a follicular pattern. Keywords: Amelolastoma; CD34; Angiogenesis
Introduction & Background of the Study
Ameloblastoma is a slow-growing tumor that aggressively invades biological bone tissue and has a high recurrence rate [1-4]. According to Mendenhall and colleagues (2007), ameloblastoma is the most frequent oral tumor emerging from odontogenic epithelial cells.
The average age of responders ranged from 42.3 to 30.4 years in Europe and Africa, respectively; however, most patients with ameloblastoma were between 30 and 60 years old [5,6]. Ameloblastoma is a rare kind of cancer in persons in their fourth and fifth decades. It is the most often occurring type of cancer in this age group [7]. The National Cancer Institute reports that the majority of patients with ameloblastoma are between the ages of 30 and 60, while the average age of respondents differs by continent and is estimated to be about 42.3 and 30.4 years in Europe and Africa, [8,9]. Pediatric ameloblastoma accounts for 10-15% of all occurrences in the general population, although this rate may be as high as 25% in Africa and Asia [10].
Although angiogenesis cannot be quantified directly, it can be inferred by examining the size of the micro vessels and the MVD of the vessels in the issue. Labeling capillary endothelial cells using monoclonal antibodies is called immunohistochemical labeling of capillary endothelial cells [11]. Antibodies to CD105, CD34, vascular endothelial growth factor (VEGF), and beta fibroblast growth factor" are just a few of the markers (monoclonal antibodies) utilized to evaluate the MVD differentiation group (CD) microscopically [12].
Ameloblastoma (AM), Odontogenic Keratocyst (OKC), and central giant cell lesions (CGCL) are all multilocular lesions with a higher prognosis than single-locular lesions (Gaonkar) [13]. CGCL is a form of odontogenic lesion that most frequently manifests as a radiolucency in the anterior mandible of the mouth in young females [14]. Multinucleated giant cell granulomas characterize mononuclear stromal cells originating within the osseous cavity and mononuclear stromal cells originating outside the cavity [15]. The CGCL exhibits a range of biological behaviors, from a progressive increase to an aggressive destructive dilatation manifested by cortical bone perforation and vascular resorption in the cortical bone [16]. AMS is the most frequent epithelial odontogenic tumor; it is locally invasive, has a high risk of recurrence, and grows continuously. Additionally, it is the most aggressive kind of tumor [17].
Acanthomatous cystic fibrosis is classified histologically into numerous subtypes. It is composed of remains of the dental plaque, cystic lining, and basal cell layer and can take the form of unicellular, multicystic, peripheral, or malignant cells. Plexiform, follicular, granular, desmoplastic, acanthomatous, and transparent cell types are among its histological forms [18]. On the other hand, malignant transformation occurs in 5% of cases and is frequently treated with marginal resection. AM with several locations’ accounts for 65% of all cases, whereas malignant transformation occurs in just 5% of cases [19-21]. Another odontogenic lesion to consider in the differential diagnosis of high recurrence AM is the odontogenic keratocyst (Keratocystic odontogenic tumor-OKC). It is derived from basal epithelial cells and exhibits a high mitotic rate associated with epithelial proliferation, superficial parakeratinization, and genetic abnormalities (p53 mutation, a tumor suppressor gene) [22]. Although OKC appears to be a cystic lesion, it acts similarly to AM regarding invasiveness and aggressiveness [23]. The examination of the behavior of these odontogenic but locally invasive lesions is centered on the microscopic epithelial and stromal features (OKC, AM, CGCL).
Even though odontogenic epithelial cells lack a circulatory system, the connective tissue stroma provides an environment conducive to epithelial transformation and expansion via angiogenesis. Epithelial cells undergo apoptosis in tumors due to a lack of nutrients and oxygen due to neovascularization. Myofibroblasts and blood vessels in the connective tissue stroma promote tumor growth, and angiogenesis is the process that occurs in these cells and arteries. Angiogenesis is the process of producing new blood vessels from existing blood vessels, and it entails several steps. Proliferation and migration of endothelial cells, proteolytic breakdown of the extracellular matrix, capillary formation, development of the loop and lumen of microvessels, and anastomosis of microvessels are all instances of bodily processes [24]. The process is regulated by growth factors and inhibitory biomarkers [25]. Although CD105 differs from normal endothelial cells (CD31 and CD34) in differential angiogenesis, an efficient angiogenesis marker should be able to distinguish between the quality and quantity of neonatal vasculature. Because angiogenesis reflects the growth potential of odontogenic tumors, evaluating mean vascular density can aid in predicting the behavior of previously examined lesions [26-27]. AM and odontogenic keratocyst (OKC) were found to have mean vascular density (MVD) values of 7.98 2.70 and 6.25 2.88, respectively, as well as a mean of 3.75 1.42, according to Kumar et al. Dentiger (Dentiger) has provided information [28]. They concluded that no statistically significant difference existed between AM and OKC (p> 0.05). According to Gadbail et al., the OKC DC has a significantly greater MVD than the regular mucosal layer (2011b). As a result, they discovered that CD105 was substantially more prevalent in odontogenic keratocyst microvessels than in normal oral mucosa or dental cyst microvessels. However, Jamshidi et al. discovered statistically significant differences in MVD between the AM and OKC groups (p = 0.005 and p = 0.000, respectively) using the CD105 and CD34 markers [28]. AM revealed a higher level of MVD than odontogenic keratocysts [29]. There appears to be a disconnect between the published research and the current understanding of the MVD of odontogenic tumors. AM, OKC, CGCL, MVD, and pyogenic granulomas are examples of odontogenic tumors that are likely to exhibit significant variation". Thus, the objective of this clinical and experimental study was to compare mean vascular density using the CD105 biomarker between adenomas, odontogenic keratocyst, and central giant cell damage samples, as well as between pyogenic granulomas, to ascertain the tumors' general behavior in the general population.
Methodology
FFPE of representative tissue was used as an inclusion criterion. At the same time, Blocks of accurate cut biopsies and non-representative tissue were excluded due to FFPE and processing artifacts.
We evaluated the H&E slides of the available blocks. The diagnosis was reconfirmed, and blocks containing sufficient tissue were chosen for IHC. Five 4-to-5-micron thin sectioned slides were prepared from representative blocks. Two were stained with H&E, two were reserved for immunohistochemistry, and one was preserved as a reserve. Additional tonsil tissue slides were put as a positive control.
MVD assessment technique:
Microscopic analysis of H&E slides was performed to confirm the diagnosis and determine which slides would be used for IHC staining. The letter was performed using a Mouse AntiHuman CD 34 Monoclonal Antibody (Clone: Q Bend 10, Product code: M7165 A/S, Glostrup, and DAKO, Denmark)."
The approach developed was used to assess the IHC staining of blood vessels in ameloblastoma [32]. The stained sections were examined at low magnification (10) to identify the most robust CD34 staining areas. Following that, a 40x magnification was used to count blood vessels. A blood vessel is a cluster of endothelial cells with a lumen; these endothelial cells are brown and positive for CD34 (CD34 positive). Three locations were chosen due to their high degree of vascularization (known as a hot spot). The MVD was calculated as the mean of three randomly chosen locations.
The following criteria were used to count the microvessels: Objects that start from a single blood vessel were counted if they are entirely detached from it [30]. Musculature-lined blood arteries were removed.
The data were analyzed statistically using the Statistical Package for the Social Sciences (SPSS) version 22. To compare MVDs, an independent t-test was performed. Statistical significance was determined by the probability value (p0.05).
Results
The study included 30 cases of ameloblastoma that had already been diagnosed. The majority of patients diagnosed with ameloblastoma were between the ages of 20 and 40 years, followed by those between 50 and 60 years, with a mean age of 36.13 + 16.0 years.
Age
The age range is between 12 and 80 years, with 36.13 + 16.0.

Figure 1: A histogram showing the mean age of the cases (n=30).
Type of Tumor
Among the 30 samples included in this study, 16 (53.3%) were follicular, 8 (26.7%) were plexiform, 5 (16.7%) were unicystic, while only 1 (3.3%) was of mixed type.
Table 1: Relationship of ameloblastoma variants with Age, Mean and standard of deviation.
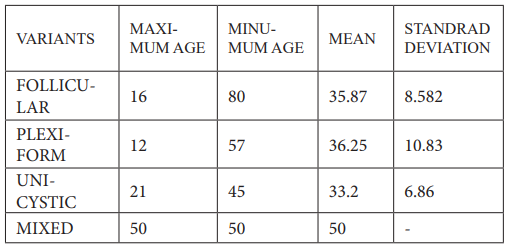
Table 2: Relationship of MVD to ameloblastoma variants.
Table 3: Age Group.

Age group: The patients were divided into following age groups:
• 1-20-years
• 21-40-years
• 41-60-years
• 61-years and above
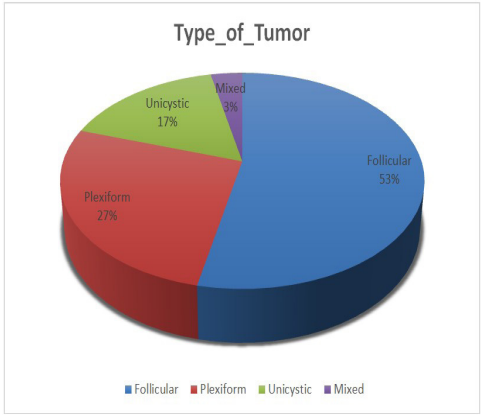
Figure 2: Frequency of the types of ameloblastoma (n=30).
Interpretation & Discussion
Since Malasses developed Adamantinoma, controversy has raged over whether treatment is the most effective at preventing the disease from recurring. Ivey and Churchill created ameloblastoma through their research [30]. Ameloblastomas are benign jaw tumors and cysts that make up about 1% of all benign tumors. The distribution is projected to be 0 copies, which equates to millions of individual years spread over the globe [5-6]. As far as we are aware, there has been a microscopic study on angiogenesis factors in CD34 patients with ameloblastoma.
The study discovered that the second and third decades of life are the most common age groups for developing ameloblastoma, with an average age of 36.13+ 16.0 years. The findings corroborated other previously published research [8-9].
Multicystic ameloblastomas in unicystosis had a greater rate of GVHD than plexiform ameloblastomas (6 cases versus 24 cases). In unicystosis, ameloblastomas with follicular ameloblastomas showed a lower rate of MVD than multicystic ameloblastomas. These findings corroborated numerous earlier studies, most notably the finding that the more aggressive a tumor derived from a particular tissue is, the higher the MVD value of that tissue is determined to be. Microvascular complications (MVD) occurred in a significant proportion of ameloblastomas, and their frequency increased with recurrence and malignant transformation [12]. The majority of odontogenic lesions have an uneven distribution of blood vessels throughout the disease. When comparing intra-tumor MVD to peritumoral MVD, ameloblastomas with intra-tumor MVD have the highest rate of angiogenesis. If there is an increase in MVD in the intravascular region, odontogenic epithelial cells are likely responsible for angiogenesis. The buildup of blood vessels next to the odontogenic epithelium to supply nutrients and oxygen plays a significant role in the rapid formation of odontogenic cysts and tumors in the mouth [31].
The most often encountered histological type in this investigation was follicular ameloblastoma (53.3%), followed by plexiform (26.7%), unicystic (16.7%), and mixed (16.7%). (3.3 percent). This is consistent with the findings of an internationally published study of common histological forms of ameloblastoma, including follicular ameloblastoma (64.9%), plexiform ameloblastoma (14.0%), desmoplastic ameloblastoma (5.2%), adenomatous ameloblastoma (5.2%), squamous metaplasia (3.9%), and unicellular ameloblastoma (1.3%) [28].
Margaritescu and colleagues hypothesized that the number of blood vessels in the peritumoral and intracranial areas are not equivalent and that this difference could be used to predict aggressive behavior and recurrence. They conducted a study to test their hypothesis [32]. MVD of SMA, UA, and desmoplastic ameloblastomas was not investigated in this study because intra-tumor and peritumoral MVD could not be compared.
Researchers have researched the age of onset of ameloblastoma, which was determined to be approximately 33-39 years. The conclusions of this analysis corroborate those of prior investigations. Additionally, they determined that sexually transmitted infections (STIs) are more prevalent among 22–26 years [32-34].
The mean age of patients with SMA, UA, and desmoplastic ameloblastoma was 32.3, 29.1, and 32.5 years, respectively, indicating that all three variants develop throughout the second and third decades of life.
In 2004, [35] assessed angiogenesis in ameloblastoma patients based on their age using CD34 antibodies. They discovered that younger age groups had plexiform ameloblastoma while older age groups had follicular ameloblastoma, indicating that angiogenesis may alter tumor growth patterns. Treatment for ameloblastoma may be further changed based on the patient's age. This study evaluated SMA, UA, and desmoplastic age and discovered that all three types of ameloblastoma, SMA, UA, and desmoplastic age, appear to be more prevalent near the second decade and the beginning of the third decade.
The mandible is anticipated to be damaged in 93.33 percent of patients (36.67 percent in SMA, 26.67 percent in desmoplastic, and 30% in UA), with the upper jaw accounting for 6.67 percent (3.33 percent in SMA and UA each). The posterior region received 76.67 percent of the total on both sides, whereas the anterior region received 23.33 percent. Based on these data, the posterior mandible appears to be the most often affected site in all three types of SMA, including those with ulcerative angiomas and desmoplastic ameloblastomatosis. This was similar to [36]. According to the findings of this study, MVD is substantially more prevalent in desmoplastic variants of SMA, UA, and ameloblastoma. When SMA and desmoplastic ameloblastoma were matched, there was also a statistically significant difference between them (P = 0.003) and UA and desmoplastic ameloblastoma (P = 0.041). demonstrates that the difference between SMA and UA was not statistically significant (P = 0.173). This is consistent with the findings of Hande et al. investigation (2011) observed that there were no statistically significant changes in MVD, total vascular area, or mean the vascular area between SMA and UA patients. This may imply that, even though SMA and UA have diverse clinical characteristics, histological presentations, and prognoses, the angiogenesis mechanism is similar in both disorders. This demonstrates that angiogenesis is critical for tumor growth and the aggressiveness of ameloblastoma.
As a result of its aggressive nature and high recurrence rate, SMA is frequently associated with UA and desmoplastic ameloblastoma, indicating that SMA is a high-risk tumor.
Researcher evaluated and compared angiogenesis in keratocystic odontogenic tumors, dentiger cysts, and ameloblastomas using a monoclonal antibody against CD34. They discovered a statistically significant difference in mean, mean MVD between the groups [37]. According to some studies, angiogenesis may be a factor in the diverse biological behavior of keratocystic odontogenic tumors, dentiger cysts, and solid ameloblastomas, all of which have been extensively investigated. Researcher reported that when benign and malignant ameloblastomas were compared to dental bacteria, greater MVD was associated with increased VEGF expression in benign and malignant ameloblastomas. They hypothesized that VEGF was involved in neoplasia, malignant transformation, or a combination of the two processes. Saif et al. studied MVD in follicular cysts, keratocystic odontogenic tumors, and ameloblastomas in 2011 using the CD34 antibody [38]. They discovered a substantial increase in mean MVD in multicystic ameloblastoma compared to keratocystic odontogenic tumors and follicular cysts. As a result, they concluded that angiogenesis is a critical process behind multicystic ameloblastoma's aggressive behavior. Additionally, this study discovered that SMA had the highest rate of MVD, followed by UA and finally desmoplastic ameloblastomas. As a result, when compared to UA and desmoplastic ameloblastoma, it is possible to conclude that SMA is aggressive.
Researcher used CD68 and CD34 antibodies to analyze the density of macrophages and microvessels associated with ameloblastomas 2012 [24]. They discovered that macrophage and microvascular densities were much more significant in SMA than in UA or desmoplastic ameloblastomas. Similarly, desmoplastic ameloblastoma patients had larger macrophage and microvascular densities than non-desmoplastic ameloblastoma patients. The findings indicate that the characteristics of these two tumor microenvironments could have a vital role in the development of ameloblastoma (a type of brain cancer). Consistent with the initial findings, this study's findings indicate a significant incidence of MVD in SMA, followed by UA and the desmoplastic type.
Researcher examined CD34 and CD105 expression in ameloblastoma and odontogenic keratocyst. Ameloblastomas had much greater levels of MVD than odontogenic keratocysts, and MVD with CD34 was significantly more prevalent in ameloblastomas than MVD with CD105 (26). Additionally, they discovered that MVD associated with CD34 was considerably more prevalent than MVD associated with CD105. As the researchers hypothesized, angiogenesis was assumed to be a factor in ameloblastoma's aggressive biological activity rather than odontogenic keratocysts.
Because angiogenesis plays a role in tumor growth, microvascular disease (MVD) is an important marker for identifying individuals with more aggressive tumors who should consider a more aggressive treatment approach. This study discovered that SMA had increased angiogenesis, followed by UA and desmoplastic ameloblastomas and that this caused aggressive behavior and a high recurrence rate for SMA.
Additionally, it is critical to evaluate the role of angiogenesis and its effect on the behavior of lesions. Histomorphometry is a technique that can be used to study and quantify the vascular area and vessel diameter of a lesion to evaluate whether or not the vascular area affects the lesion's aggressive behavior. Researcher and colleagues [34] developed this method in their research. This study did not investigate the size of the vascular area and its role in the lesion's aggressive behavior.
The measurement of tumor proliferative and invasive activity may aid in predicting the malignancies' biological behavior. As a result, treatment decisions and recurrence risk are considerably influenced.
The study discovered that all three variations occurred in young people, that men were more frequently afflicted than women, and that the lower back was the most frequently impacted area.
This investigation discovered a statistically significant association between MVD of all three forms, namely SMA, UA, and desmoplastic ameloblastoma, in all three cases. When the three variants were paired, a significant relationship between SMA and desmoplastic ameloblastoma and UA and desmoplastic ameloblastoma was seen (Figure 1). However, no statistically significant association between SMA and UA was discovered. This may imply that, even though SMA and UA have diverse clinical characteristics, histological presentations, and prognoses, the angiogenesis mechanism is similar in both disorders.
The findings of this study reveal that while angiogenesis plays a role in the growth and aggressiveness of ameloblastomas, it is neither a strong predictor nor a precise diagnostic test for the three variants examined: SMA, UA, and high MVD in desmoplastic ameloblastomas. This study lays the groundwork for future research into the absolute utility of angiogenesis in ameloblastomas, which will utilize CD34 as an immunohistochemical marker. To begin, additional research is necessary, which should include patient monitoring. Additionally, the actual value of MVD as an independent prognostic measure in ameloblastomas must be determined.
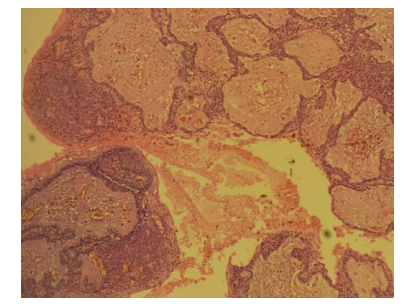
Figure 3: The follicular ameloblastoma (H &E 10X) arrow shows the vessel.
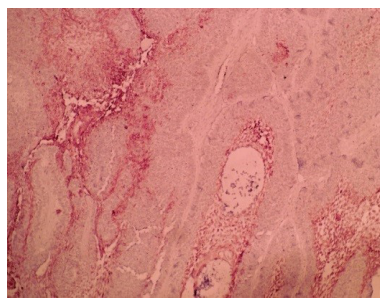
Figure 4: Microvessels positive for immunohistochemical staining of CD 34 antigen for follicular ameloblastoma (10X).

Figure 5: Microvessels positive for immunohistochemical staining of CD 34 antigen for plexiform ameloblastoma (10X).
Conclusion
Ameloblastoma was most prevalent in the second and third decades of life. This study provided critical insight into the role of angiogenesis in ameloblastoma variants, follicular showed higher MVD as compared to plexiform, Unicystic showed lesser MVD as compared to multicystic ameloblastoma. younger patients are more likely to develop a plexiform form of ameloblastoma than older patients, and that elderly patients are more likely to develop a follicular pattern. Unicystic is more common in young age. As a result, by categorising patients according to their age, we can identify variables that may influence the future growth pattern of ameloblastoma.
Research Limitations
1. Due to the small sample size, our research may not represent the entire population.
2. Blocks and slides for laboratory record-keeping are not up to grade.
3. Inadequate coordination between the surgeon and pathologist is a significant issue.
4. The primary analysis factors (processing and approval time) were not uniform because the samples were collected from various locations.
References
- Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol, 1995; 31B: 86-99.
- Ruiter D, Bogenrieder T, Elder D, Herlyn M. Melanoma-stroma interactions: Structural and functional aspects. Lancet Oncol, 2002; 3: 35-43.
- van den Hooff A. Stromal involvement in malignant growth. Adv Cancer Res, 1988; 50: 159-196.
- Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol, 2001; 11: 97-104.
- Coussens LM, Werb Z. Inflammatory cells and cancer: Think different! J Exp Med, 2001; 193: F23-26.
- Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis – correlation in invasive breast carcinoma. N Engl J Med, 1991; 324: 1-8.
- Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med, 2003; 3: 643-651.
- Kumamoto H, Ohki K, Ooya K. Association between vascular endothelial growth factor (VEGF) expression and tumor angiogenesis in ameloblastomas. J Oral Pathol Med, 2002; 31: 28-34.
- Naumov GN, Bender E, Zurakowski D, Kang SY, Sampson D, Flynn, et al. Amodelofhumantumordormancy: Anangiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst, 2006; 98: 316-25.
- Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia: Elsevier Saunders Co. 2005.
- Pandiar D, Shameena P. Immunohistochemical expression of CD34 and primary fibroblast growth factor (bFGF) in oral submucous fibrosis. J Oral Maxillofac Pathol, 2014; 18: 155-161.
- Kademani D, Lewis JT, Lamb DH, Rallis DJ, Harrington JR. Angiogenesis and CD34 expression are predictors of recurrence in oral squamous cell carcinoma. J Oral Maxillofac Surg, 2009; 67: 1800-1805.
- Lanza F, Healy L, Sutherland DR. Structural and functional features of the CD34 antigen: An update. J Biol Regul Homeost Agents, 2001; 15: 1-13.
- Li SH, Hung PH, Chou KC, Hsieh SH, Shieh YS. Tumor angiogenesis in oral squamous cell carcinoma: The significance of endothelial markers and hotspot selection. J Med Sci, 2009; 29: 67-74.
- Hande AH, Gadbail AR, Someone AM, Chaudhary MS, Wadhwan V, Nikam A. Comparative analysis of tumor angiogenesis in solid multicystic and unicystic ameloblastoma using CD 105 (endoglin). Arch Oral Biol, 2011; 56: 1635-1640.
- Frangou EM, Lawson J, Kanthan R. Angiogenesis in male breast cancer. World J Surg Oncol, 2005; 3: 16.
- Segatelli V, de Oliveira EC, Boin IF, Ataide EC, Escanhoela CA. Evaluation and comparison of microvessel density using the markers CD34 and CD105 in regenerative nodules, dysplastic nodules, and hepatocellular carcinoma. Hepatol Int, 2014; 8: 260-265.
- Chaloob MK, Ali HH, Qasim BJ, Mohammed AS. Immunohistochemical expression of Ki-67, PCNA and CD34 in astrocytomas: A clinicopathological study. Oman Med J, 2012; 27: 368-374.
- Ancuta C, Ancuta E, Zugun-Eloae F, Carasevici E. Neoangiogenesis in cervical cancer: Focus on CD34 assessment. Rom J Morphol Embryol, 2010; 51: 289-294.
- Rossochacka-Rostalska B, Gisterek IJ, Suder E, Szelachowska JK, Matkowski RA, Lacko A, et al. Prognostic significance of microvessel density in ovarian cancer. Wiad Lek, 2007; 60: 129- 137.
- Mohtasham N, Babakoohi S, Salehinejad J, Montaser-Kouhsari L, Shakeri MT, Shojaee S, et al. Mast cell density and angiogenesis in the oral dysplastic epithelium and low- and high-grade oral squamous cell carcinoma. Acta Odontol Scand, 2010; 68: 300-304.
- Scardina GA, Ruggieri A, Maresi E, Messina P. Angiogenesis in oral lichen planus: An in vivo and immunohistological Pereira et al. 58 evaluation. Arch Immunol Ther Exp (Warsz), 2011; 59: 457-462.
- Seifi S, Shafaie S, Ghadiri S. Microvessel density in follicular cysts, keratocystic odontogenic tumors, and ameloblastomas. Asian Pac J Cancer Prev, 2011; 12: 351-356.
- Taher MG, Abdullah BH, Al-Khuri LE. Immunohistochemical expression of CD34 as a biological marker of angiogenesis and expression of D2-40 as a marker of lymphangiogenesis in mucoepidermoid carcinoma of salivary glands. J Pak Med Stud, 2012; 2: 126-133.
- Neville BW, Dam DD, Allen CM, Bouquet JE. Oral and Maxillofacial Pathology. 2nd ed. Philadelphia: WB Saunders; 2002; p. 611-619.
- Jamshidi S, Zargaran M, Baghaei F, Shojaei S, Zare Mahmoodabadi R, Dehghan A, et al. An immunohistochemical survey to evaluate the expression of CD105 and CD34 in ameloblastoma and odontogenic keratocyst. J Dent (Shiraz), 2014; 15: 192-198.
- Wang A, Zhang B, Huang H, Zhang L, Zeng D, Tao Q, et al. Suppression of local invasion of ameloblastoma by inhibition of matrix metalloproteinase-2 in vitro. BMC Cancer, 2008; 8: 182.
- Ribeiro BF, Iglesias DP, Nascimento GJ, Galvão HC, Medeiros AM, Freitas RA. Immunoexpression of MMPs-1, -2, and -9 in ameloblastoma and odontogenic adenomatoid tumor. Oral Dis, 2009; 15: 472-477.
- Iezzi G, Piattelli A, Rubini C, Artese L, Goteri G, Perrotti V, et al. Expression of transforming growth factor beta1 in ameloblastomas. J Craniofac Surg, 2008; 19: 1618-1621.
- de Medeiros AM, Nonaka CF, Galvão HC, de Souza LB, Freitas Rde A. Expression of extracellular matrix proteins in ameloblastomas and adenomatoid odontogenic tumors. Eur Arch Otorhinolaryngol, 2010; 267: 303-310.
- Vered M, Shohat I, Buchner A, Dayan D. Myofibroblasts in the stroma of odontogenic cysts and tumors can contribute to variations in the biological behavior of lesions. Oral Oncol, 2005; 41: 1028-1033.
- Margaritescu C, Pirici D, Stînga A, Simionescu C, Raica M, Mogoanta L, et al. VEGF expression and angiogenesis in oral squamous cell carcinoma: An immunohistochemical and morphometric study. Clin Exp Med, 2010; 10: 209-214.
- Ackermann GL, Altini M, Shear M. The unicystic ameloblastoma: A clinicopathological study of 57 cases. J Oral Pathol, 1988; 17: 541-546.
- Lau SL, Samman N. Recurrence related to treatment modalities of unicystic ameloblastoma: A systematic review. Int J Oral Maxillofac Surg, 2006; 35: 681-690.
- Koizumi Y, Kauzman A, Okada H, Kuyama K, McComb RJ, Yamamoto H. Assessment of proliferative activity and angiogenesis in ameloblastoma. Int J Oral Med Sci, 2004; 3: 25-33.
- Barnes L, Eveson JW, Reichart P, Sidranksy D. Classification of Tumors. Pathology and Genetics of Head and Neck Tumors. Lyon: IARC Press; 2005; p. 283-328.
- Alaeddini M, Salah S, Dehghan F, Eshghyar N, Etemad-Moghadam S. Comparison of angiogenesis in keratocystic odontogenic tumors, dentigerous cysts and ameloblastomas. Oral Dis, 2009; 15: 422-427.
- Guzmán-Medrano R, Arreola-Rosales RL, Shibayama M, Silva-Olivares DA, Bologna-Molina R, Rodríguez MA. Tumor-associated macrophages and angiogenesis: A statistical correlation that could reflect a critical relationship.

