Use of Organoids for anticancer Drug Development
Tripathi K, Lazarevic M*
Internal Medicine, SMT NHL Medical Municipal College, India
Internal Medicine, Swedish hospital, USA
Received Date: 17/05/2022; Published Date: 27/05/2022
*Corresponding author: Dr Kaushalendra Tripathi, Internal Medicine, Swedish hospital, Chicago, Illinois, USA
Abstract
Much of organoid research has changed the way developmental neuroscience works, providing unprecedented access to human neurodevelopment and function [1-8]. Figure 1 illustrates the timeline in which the Neural Stem Cells (NSC) were cultured [9]. Glioblastoma is a highly malignant brain tumour with significant intratumor heterogeneity, which could be ascribed to Glioblastoma Stem Cells (GSC) activity variations. Using a variety of experimental techniques including quantitatively evaluating lineage trace, clonal size, mutational marker evaluation, and single cell RNA sequencing showed a growing accretion in GBM and outlined the outcome of tumour cells is regulated by a neurogenesis developmental pathway [10-13].
Keywords: Brain organoids; GBM; Drug development
Introduction
Inhibiting the stem cell could also limit tumour recurrence in another brain tumour that originated in the cerebellum [14]. With the application of the above-mentioned studies, the Cancer Stem Cell (CSC) model displayed a structure for understanding tumour variation, predicting tumour progression, and potentially assisting in the development of new therapeutics. There exists a duality in GSC with it displaying tumor heterogeneity when replicating in culture or xenotransplantation, which makes it difficult to treat. While utilizing 2D monolayer cultures did not yield variation and 3D relative spatial organization displaying a lack of interaction with various the cancer extra cellular matrix and microenvironment. Furthermore, they may not accurately determine therapeutic effectiveness, as medications that first demonstrated promising results in cultured cell lines did not find their way into clinical trials. Hence more sophisticated model frameworks that can replicate complex cancer characteristics while enabling exhaustive examination are necessary. Particularly in light of the necessity to provide more precise forecasts for novel therapeutic benefits. Successful results in other cancer disciplines have incentivized several laboratories to generate organoid glioblastoma models consisting of 3D self-organizing structures, allowing particular cell-cell contacts leading to a generation of a specific microenvironment [15-19]. Consequently, GBM organoids could tumor complexity and variation in their proliferative capacity and response to treatment.
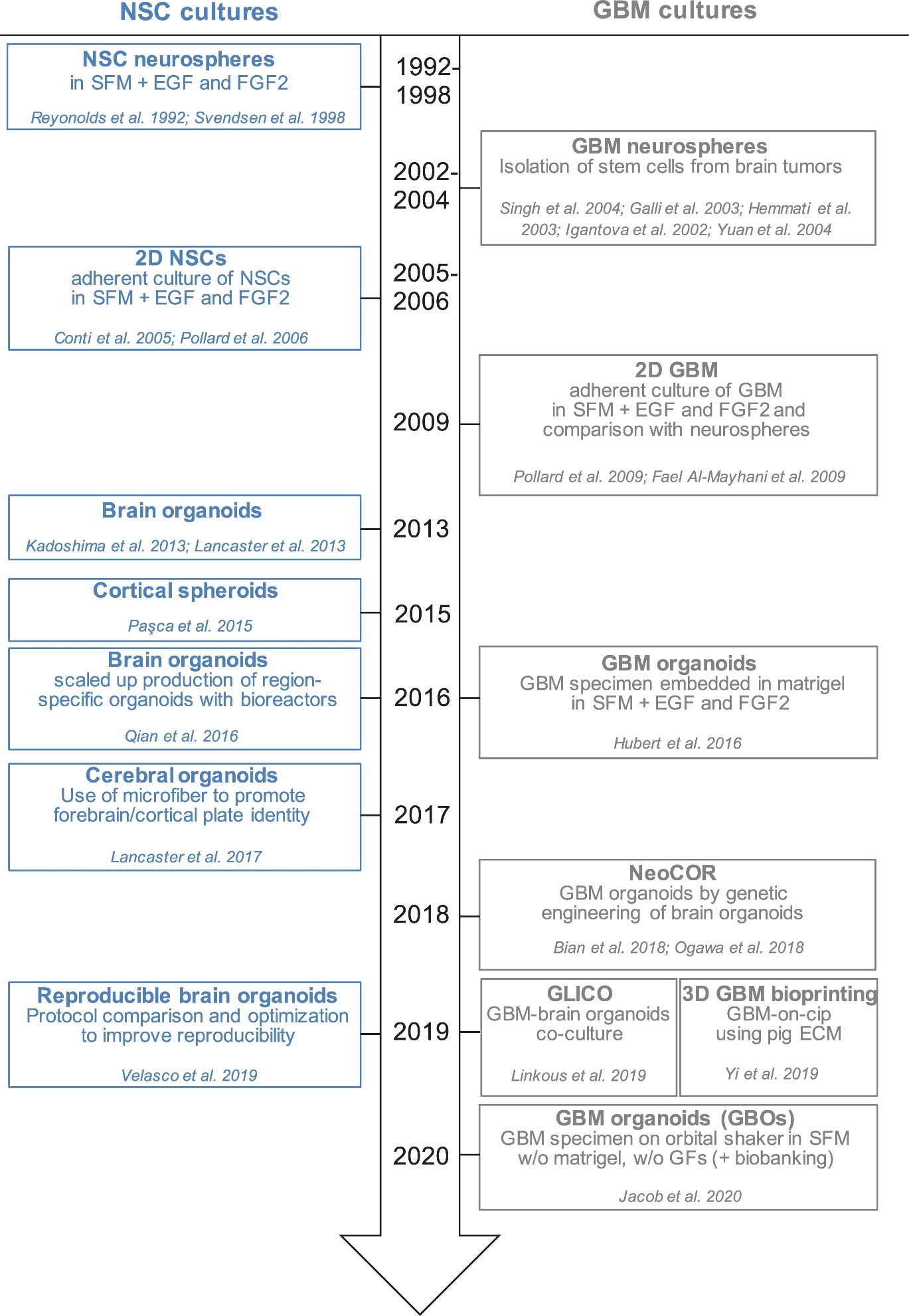
Figure 1: Timeline of in vitro method to culture neural stem cells.
Discussion
Glioblastoma stem cells are derived from tumor tissues and cultivated long durations in culture [20-28]. EGF (Epidermal Growth Factor) and FGF2 (Fibroblast Growth Factor) maintain GSC development in vitro [29-31]. GSC is produced in 2D or 3D cultures, regarded as a first “3D model” because the cells preserve polarisation and 3-dimensional spatial configuration [32,33]. Neurospheres, on the other hand, have a necrotic foundation and can grow to a peak value of roughly 300 m before requiring breakage and replating to thrive [34-36]. Furthermore, cells in neurospheres lack their contact with extracellular matrix proteins, hence do not closely resemble GSC behavior in vivo. Jürgen Knoblich and Inder Verma genetically modified organoids to grow malignancies lately [37,38]. Bian et al. (2018) looked for changes leading to cancer and called them NeoCor (neoplastic cerebral organoids): the authors used a transposase-based approach to overexpress recognised oncogenes and/or used CRIPSR Cas-9 to remove tumour suppressor gene activities. Organoid cells targeted with nucleofection at an early stage of development, and the cells containing the genetic modifications were labelled with GFP, permitting cell proliferation and tumour development to be monitored precisely. MYC overexpression, as well as a few other genetic sequences induced proliferation. Transcriptome profiling revealed that tumors with MYC possess CNS-PNET-like identity (CNS-PNET: Primitive Neuro-Ectodermal Tumor), whereas different alternative tumors possess a GBM-like identity, implying that different anomalies can cause tumours that possess unique markers. Researchers have discovered that certain genetic alterations, such as HRasG12V activation and p53 disruption, can produce mesenchymal GBM in organoids. Although these studies suggest that some GBM subtyping can be cloned, it remains to be seen whether all GBM subtypes could be cloned with the technology and how much the GBM-derived organoids reflect patient-derived GBM cells [9].
Because they more precisely mimic the complexity and heterogeneity of a natural tumour, 3D in vitro models are promising for studying GBM biology and predicting treatment response. In fact, the majority of the models presented depict selected vulnerabilities to pharmaceuticals or radiation, which mimic tumour sensitivity in vivo [37,39,40]. Furthermore, the creation of a live GBM biobank has been facilitated by newer generation of 3D GBM organoids using a new and quicker methodology (one to two weeks). Some models discussed in this study [37,38,40-42] allow non/GBM brain cells to be combined together. This is particularly valuable for Investigating the interaction between tumor infiltration into normal tissue and tumor brain cells. It is possible to study the exact role of genes involved in cell-cell interaction, adhesion, guidance, and migration. This may lead to the discovery of new therapeutic targets which inhibit tumor infiltration. These 3D GBM organoid models advance the study of cancer stem cell variation by providing cancer cells with an environment in which they can maintain the coexistence of different stem and progenitor cells [43]. They will also allow researchers to investigate the developmental hierarchy of CSCs in malignancies, as well as the impact of other cell types or the microenvironment on CSC fate decisions. Cells such as microglia or other immune cells, can be incorporated into organoids [44-47]. The key problem is to replicate an environment with the vasculature and other cell types that can display inflammatory and immune described similarly to those seen in a healthy brain (48-50). The presence of multiple cell types and greater diversity is probably the greatest strength and weakness. Because while they reflect the complexity of the primary tumor, they are also the source of the variations in a 3D environment [51-55]. Thus, depending on whether they want to undertake bulk analysis on a homogeneous cell population, investigators may have to select amongst growing cells in traditional 2D monolayer or spherical cultures or 3D organoids.
Some model kinds are as follows: Glioma Spheroid (GS) is a kind of glioma (with serum) cancer stem cell growth is not especially encouraged in a dense conglomeration of cells grown in serum. Glioma tumouroid tumour organoids were created by culturing primary tumour material in suspension in the absence of serum under specific media conditions, with CSCS specifically promoting cellular heterogeneity and maintaining cellular heterogeneity. Brain Organoid (BO) is a model organ derived from stem cells grown in particular medium and under specified growth circumstances to encourage tissue lineage development. It has some of the functions and physical characteristics of a natural organ. GS/BO and GT/BO are two different types of GS/BO. Single cells, spheroid, and tumoroids supported in liquid solution. Matrix-supported Single cells, spheroid, or organoid encased in a three-dimensional matrix. Below is a table describing models and their corresponding findings [56]
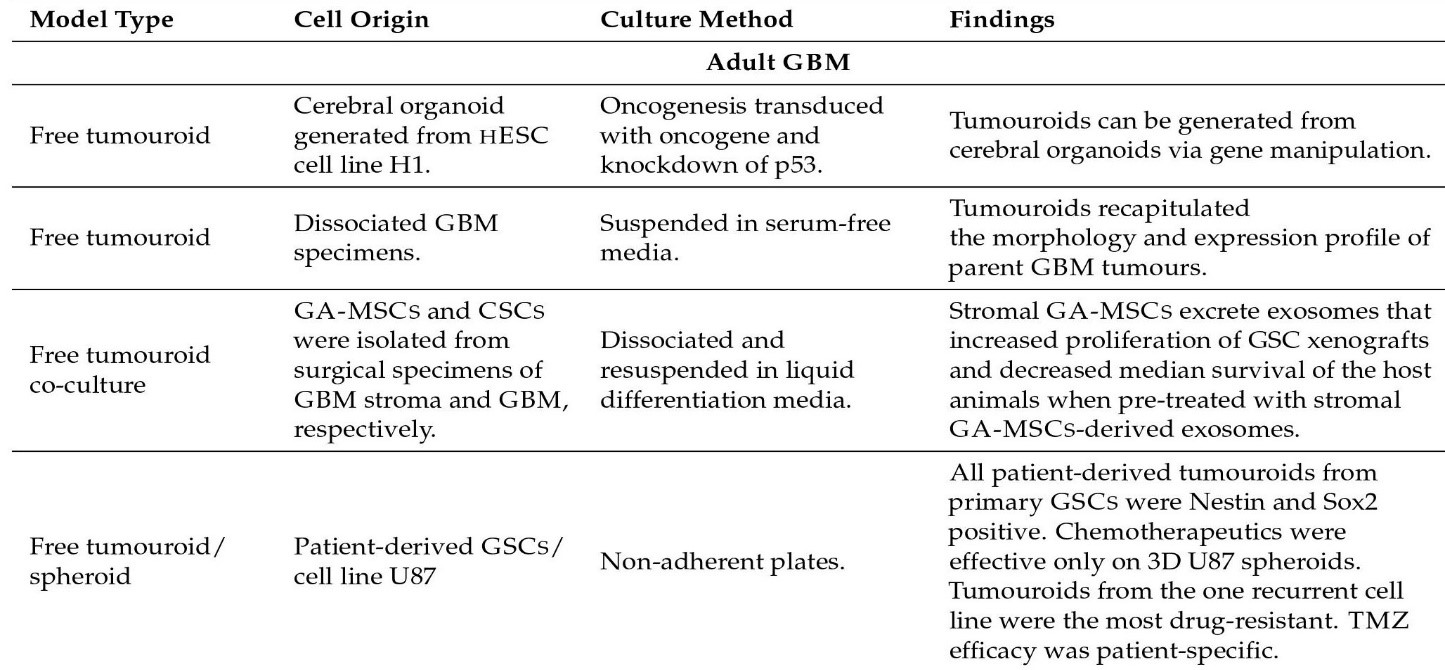
Figure 2: Organoid models and their findings.
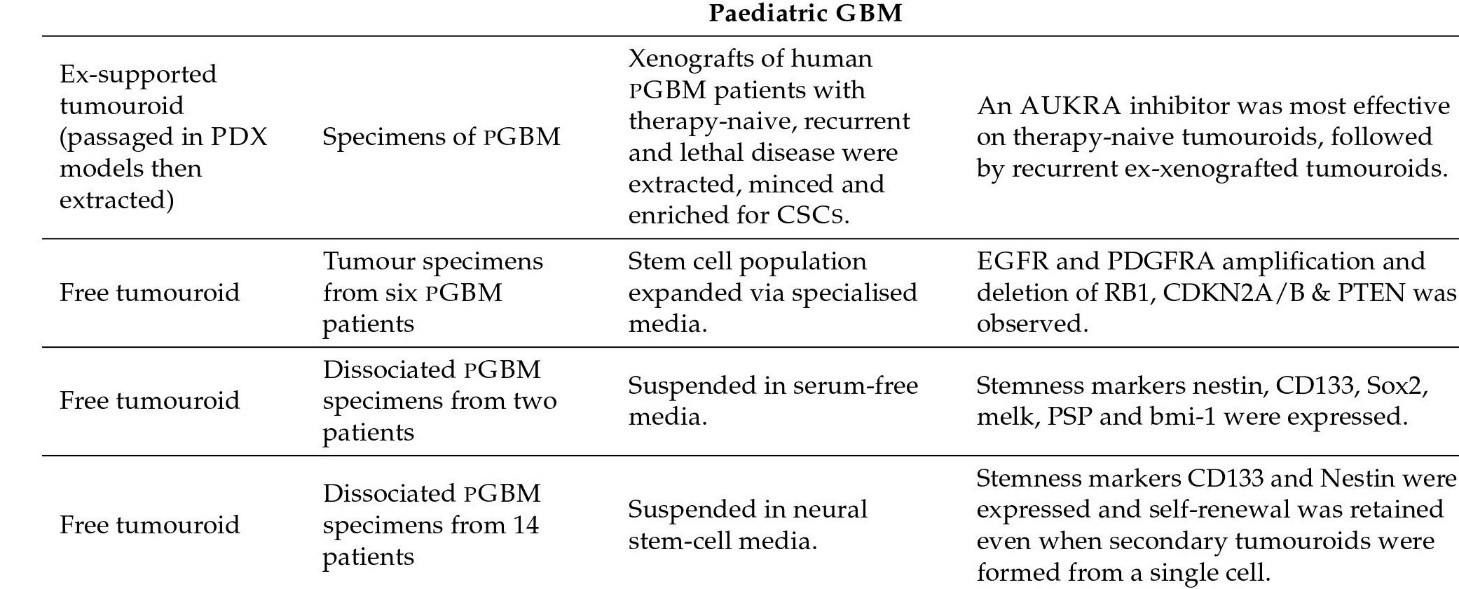
Figure 3: Pediatric Organoid models and their findings.
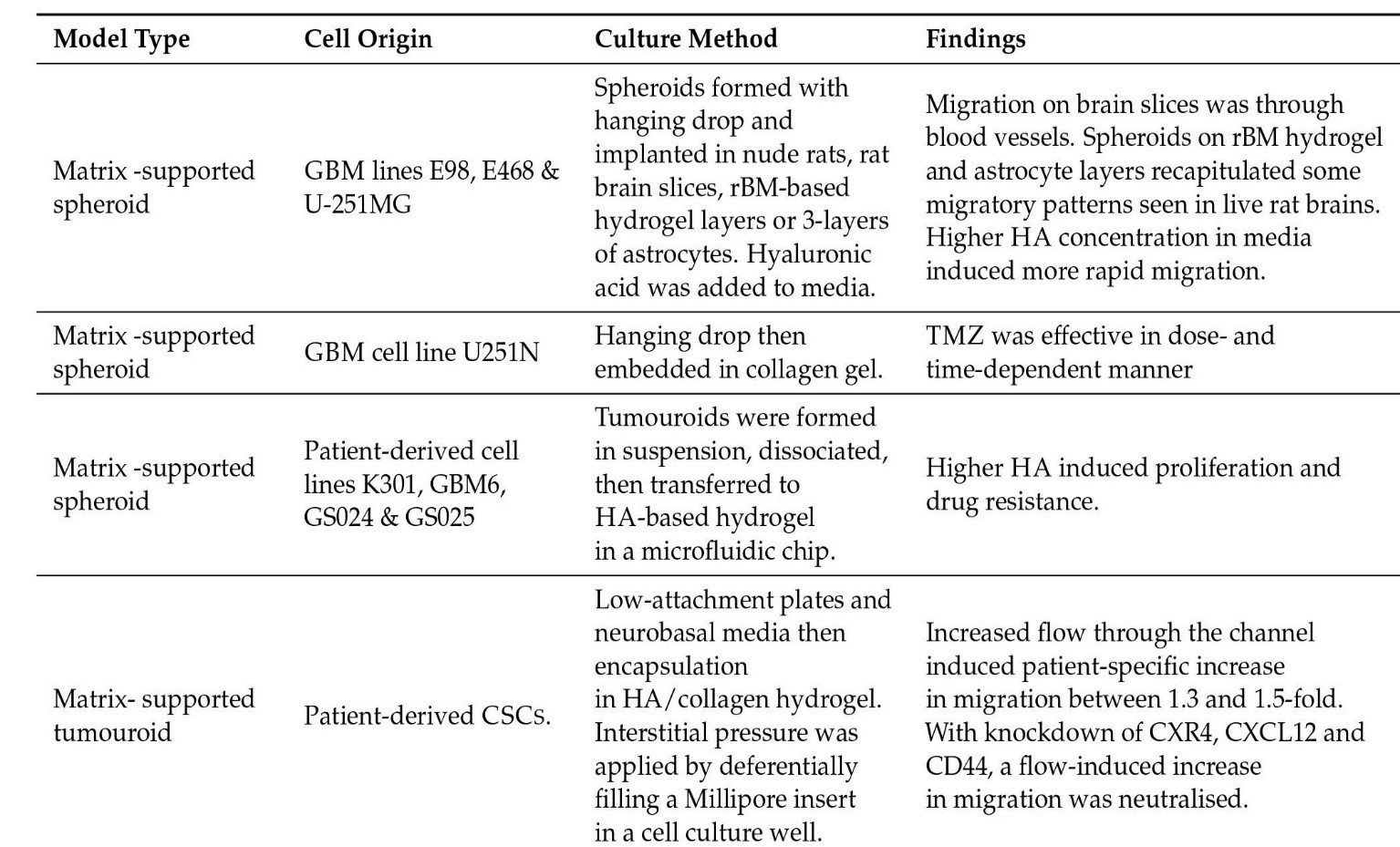
Figure 4: Matrix supported models and their findings.

Figure 5: Brain Organoid models and their findings.

Figure 6: Co-culture models and their findings.
Bioprinting is a type of matrix-supported cell culture in which cell-loaded bioink is applied to a print bed with a custom-made 3D printer. The two most common processes are extrusion (filament) and inkjet (droplet). Inkjet printing is a non-contact method that uses heat or electricity to eject bioink droplets from nozzles and can also be performed using a dedicated office printer. The extrusion pressure is either pneumatic or a piston that applies pressure to the bioink reservoir and pushes it into the nozzle. You can then use the CAD file to modify the nozzles to create a precisely controlled bio-ink pattern. Individual cells can be manipulated and placed individually using a laser-based printing technique known as laser-based direct writing. Bioinks are hydrogels with living single cells or cell aggregates (spheroids) or cancerous tumors. Using 3D printing for layered gel structures gives you unparalleled control over the distribution patterns of different cell / gel structures. The first bioprinted cell / hydrogel structure was made with HeLa cells and gelatin / alginate / fibrin bioink. Below is a table that describes the bioprinted culture and its corresponding results [56].

Figure 7: Bio-printed models and their findings.

Figure 8: Illustration of bioprinting technology and the two types of gels that are used.
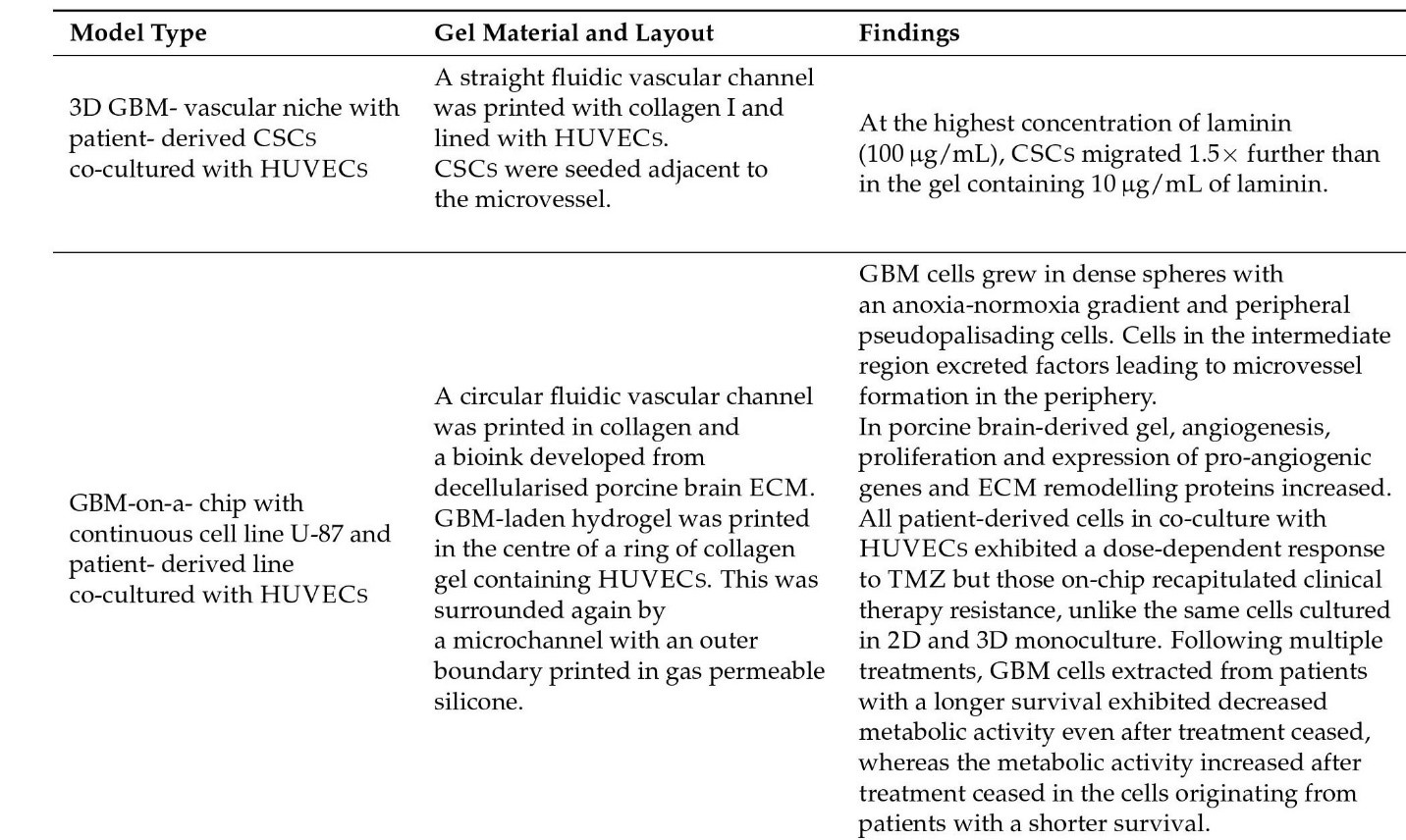
Figure 9: Vasculature models and their findings.
Driehuis et al. created an organoid biobank (N = 31) from head nd neck squamous cell carcinoma (HNSCC). Patient Derived Organoids (PDO) mimic the parental HNSCC and creates tumors when transplanted into immunocompromised mice. Various reactions were noticed by drugs used in a clinical setting. These drugs included cisplatin, carboplatin, cetuximab, and in vitro radiation therapy. In addition, drug screening demonstrates reactivity to pharmaceuticals not routinely used in clinics for patients with HNSCC. The results could individualize HNSCC treatment and expand the HNSCC drug portfolio. In another study, the authors reported that HNSCC-derived PDO are able to be utilized to study reactions to photodynamic therapy, while simultaneously testing it on corresponding normal tissue-derived organoids to ensure the safety of treatment [57,58].
On drug sensitivity and resistance testing, Clear Cell Ovarian Cancer (CCC1) organoids were resistant to paclitaxel, cisplatin and carboplatin in comparisoon to alternative organelles. This is due to the fact that clear cell ovarian cancer is resistant to platinum-based chemotherapy (response rate: 11.1% clear cell, 72.5% serous). CCC1 has a mutation in the SWItch/Sucrose NonFermentable (SWI/SNF)-associated gene; PBRM1 (p.P1460L) and ARID1A (p. P1995Lfs * 22, p. Q1098Rfs * 16) implying that blocking the immune checkpoint could be an approach. High grade serous cancer cells (HGSC) called HGSC1 and HGSC3 patients have CNV-like HRD, whereas HGSC2 are restricted with CNV. HGSC1 and HGSC3 exhibited sensitivity to paclitaxel treatment; However, HGSC2 was resistant. HGSC1 contains the variant BRCA1, hence it is sensitive to PARP inhibitors, olaparib and cisplatin relative to other organelles. Both HSGC1 and HGSC2 are from FIGO stage IIIC tumors. The duration at which there was no disease after platinum treatment was lengthier in HGSC1 compared to HGSC2 [59]. Kopper et al. stated in vitro drug susceptibility was reproduced in vivo by xenotransplantation of ovarian cancer organoids [60].
Because drug responses are varied and better correlated with genomic changes in 3D culture than in 2D culture. Organoids are a suitable culture format for drug susceptibility analysis in translational studies [61]. Disadvantages of the organoid model include the absence of cancer stroma such as fibroblasts, blood vessels, and immune cells. However, recently there have been reports of gas-liquid interface methods that maintain the microenvironment of the tumor immune system [62].
Esophageal adenocarcinoma organoids have been treated with standard chemotherapy (5FU, epirubicin, and cisplatin). All patients except one had a meager reaction to chemotherapy, and the organoids of the patients who displayed a response were not obtainable for testing [63]. A common response in the organoid and tumor reactions of four patients to chemotherapeutic agents (cisplatin, paclitaxel, 5FU, epirubicin, and irinotecan) was noted in another report [64]. In a report, gastric cancer organoids obtained from patients prior to treatment were sensitive to standard chemotherapy (5fluorouracil (5FU), cisplatin, oxaliplatin, and irinotecan), despite the contribution of radiation therapy. It reproduced the patient's complete pathological response after chemotherapy, though the clinical response due to the radiotherapy had not been thoroughly examined [65]. An additional study found inconsistent outcomes with a combination of 5FU, oxaliplatin, and epirubicin with seven gastric cancer patients mentioned in this report, only two patients were found to correlate with drug reactions (5FU, oxaliplatin and epirubicin combination). A clinical response that corresponded with an organoid response was only recorded in one patient [66].
Gastric cancer organoids derived from ascites displayed a varied respond to chemotherapeutic agents (oxaliplatin, 5FU, cisplatin, docetaxel, Irinotecan, epirubicin, and paclitaxel) among patients, which also had synonymous heterogenous responses with peritoneal metastasis [67].
Colorectal cancer organoids were utilized in cases who would take advantage of the cross-sensitivity to olaparib and oxaliplatin. This induces PARP-dependent DNA damage repair. In two patients who reacted to oxaliplatin, the organoids responded well to olaparib and oxaliplatin. In a different case who responded, the organoid was resistant to therapeutics. The organoids in this patient reacted particularly strongly to panitumumab. Panitumumab was utilized in treatment and may be a major contributor to the clinical response, elucidating inconsistencies among organoids and reaction [68].
Organoids representing metastatic GI cancers had sensitive reactions with cetuximab (anti-EGFR monoclonal antibody), but possessed resistance in patients with EGFR amplification and KRAS wild-type [69].
Rectal cancer organoids underwent treatment with standard chemotherapeutics (5FU alone or FOLFOX (5FU, leucovortin and oxaliplatin) or radiation therapy (one dose, 08 Gy). An association of 5FU or FOLFOX (r = 0.86) was detected in relation to Progression-Free Survival (PFS) in seven patients. In the case of radiation therapy, the organoids that were resistant to radiation therapy originated from already irradiated tumors or tumors without or negligible responses. Radiosensitive organoids originated in cases which had a reduced tumor size by a minimum of 50% or those who displayed complete clinical remission after radiation therapy. In addition, organoids (N = 80) that underwent neoadjuvant chemoradiotherapy such as 5FU and irinotecan indicated predictions of remission to treatment with chemotherapeutics (sensitivity 78.01%, specificity 91.97%) [70,71].
Everolimus was identified as a therapeutic candidate for patients which had GBM organoids obtained from them and elicited an incomplete reaction [72]. Cancer organoids in GBM patients had also been tested for susceptibility to standard chemotherapy temozolomide and agents directing towards mTOR, PI3K, or DNA damage responses. Varied reactions to monotherapy and the combination of temozolomide with targeted drugs has been detected between organoids in diverse cases [73].
A CNS carcinoma, chordoma organoid possessed PD1-positive CD8 T cells and was utilized to establish a connection with a nivolumab reaction (PDL1 blockade). Patients with PDL1-positive and PDL1-negative tumors had dose dependent reactions. This is consistent with observations that under expressed PDL1 may possess a response to PDL1 inhibition. It can be inferred that response to the therapy of cancer organoids may be foreseeable irrespective of PDL1 standing, but an association with distinct patient responses was not established. 24 patients with primary chordoma cases at the G. Pascal and G. Pini Foundations. Two monoclonal antibodies were utilized to stain surgical samples against PDL1, E1L3N, and 288 (Cell Marque) with the BenchMark XT kit and an automated immunostainer Ventana Medical Systems, as directed by the manufacturer. PDL1-positive cancer cells and lymphocytes were determined as the percentage of positive cells in all sections according to FDA guidelines. The median age in the study was sixty-five years with a range of fifty-five to seventy-nine. The average period for a follow up was usually six months or higher. Twelve patients had passed away and the survival period was at a median of fifty months. Immunotherapy aiming automatic cell death 1 receptor (PD1) and its ligand 1 (PDL1) produced remarkable outcomes in progressive cancers showing increased expression of PDL1,1,2. Displaying exemplary therapeutic effect but a lack of patients for trials do not assist in concretely proving therapeutic uses. This is especially significant for chordomas, rare malignancies that are located primarily on the spinal axis, have an elevated recurrence rate (43-85%). They are less prone to distant metastases. chondroma is a candidate for immunotherapy because it expresses more PD1 / PDL1 than healthy bone tissue though it is resistant to chemo and radiotherapy. Clinical trials investigated the efficacy of PDL1 targeted therapy with nivolumab only or with ipilimumab. Studies on the combination of nivolumab and stereotactic radiosurgery have also been conducted. From a clinical point of view, the selection of cancers / patients who are sensitive to anti-PDL1 blocking therapy based on PDL1 expression in tumor cells and lymphocytes that infiltrate tumors would be extremely useful. The antibodies reacting to PDL1 was evaluated the expression of all surgical specimens of tumor cells and tumor-infiltrating lymphocytes distinctly, comparing the results with clinical limits. As the potential benefits of organoids for cancer cell culture are increasingly recognized, organoids from patients are also produced, quantifying diameter, cell death and PDL1 presence to determine the dose-dependent effect of nivolumab. This helped establish a treatment response and method [74].
Several strains of organoid Retinoblastoma have been produced and underwent standard chemotherapy (melphalan, topotecan, and methotrexate) and display similar responses to tumor cells in cases with progressive disease. Though no direct correlation to patient reaction was ever established. Lasting or recurring retinoblastoma (RB) is related with vitreous and/or in subretinal mets in progressive RB and is a primary reason for treatment failure. This requires the improvement of new therapeutics and, therefore a cutting-edge RB model for testing therapies. The authors created a three-dimensional self-assembling organoid model derived from chemo sensitive tumors. They established and equated the response of organoids to drugs and associated the organoid model with advanced RB in terms of drug susceptibility. Organoids show histological characteristics suggestive of retinal tumors and seeds, and have been found to retain changes in DNA copy count and gene and protein manifestation from the parent tissue. Cone signaling circuits (M / L + cells) and glial tumor microenvironments (GFAP + cells) were predominantly existing in organoids. Topotecan isolated or a combination of topotecan and melphalan successfully targeted the organoid cancer cones (RXRγ + Ki67 +) and blocked the invasion of mitosis after 24 hours of therapeutic exposure. In comparison, MTX was slightly effective in treating cancer cells. The reaction of organoids was constant with that of cancer cells in progressive disease. Organoids from patients lead to the production of models for use in exploring new therapies for use in retinoblastoma.
Drugs clinically used for intravitreal chemotherapy (melphalan, topotecan, and methotrexate) were treated to determine if the advanced RB drug response was reproduced in organoid cultures (RB688). In addition, comparisons have been made between combination drugs (melphalan and topotecan) and individual drug regimens, making systematic implementation in the clinic an issue. The drug concentration used in this study was comparable to the levels in the vitreous cavity. Since tumor organoids have a cell structure similar to that of tumor tissue. It has been shown that the accessibility and uptake of the active ingredient occurs in the deepest regions of the tumor organoid core. This is due to the increase. ΓH2AX lesion, which is a DNA damage reaction marker [75].
Copper’s influence on brain development and function is documented. Unfortunately, minute information is available on how Copper works mechanically in certain central nervous system processes. Cultures are available to make brain organoids also known as cerebral globules that contain cell types that mimic multiple areas of the brain. As far as it is known, the neurobiology of Cu-based disorders in brain-derived organoids has not yet been investigated. Brain organoids were tested for Cu content using X-ray fluorescence microscopy technology. This showed low copper in the areas scrutinized [76].
When we are enhancing cancer treatment for an individual patient, it is crucial to advance the effect of the cancer treatment on the tumor tissue and to personalize it. The current main problems with current chemotherapeutic agents are the harmful side effects leading to the restricting their use over time and finding the right dose. In order to defeat the inadequate rate of new therapies that progress through the stages of the clinical trials, improved processes for establishing serious side effects in the preclinical stage are desirable. The use of non-transformed organoids obtained from tumor tissue enables the ability to test the specificity of therapeutic agents [77,78]. Moreover, organoids originating from tissues primarily disturbed by side effects, used to proactively identify and explore potential problems. The central nervous system organoids are utilized to evaluate neurotoxicity. In this regard, Liu et al. evaluated the toxic effects of vincristine on brain organoids. Dose-dependent toxicity to both neurons and astrocytes was revealed. In addition, Schielke et al. depicted the benefits of utilizing brain organoids to improve radiation therapy for CNS tumors [79,80,81,82].
Conclusion
There is a greater need to explore and study ideas the use of organoids, this will lead to a greater increase in life span and 5-year survival rates. Classic cancer cell lines and animal cancer models are physiologically and clinically more established than patient-derived tumor organoids. Furthermore, as compared to traditional cancer cell lines and PDX models, patient-derived tumor organoids are more capable of capturing and retaining the molecular, cellular, genetic, and histological characteristics of the tumor of origin while also preserving patient-specific tumor heterogeneity. Despite the obstacles ahead, human organoids offer a lot of potential in cancer treatment. With the rapid advancement of other technologies, we believe that synergistic applications using organoids can help bridge the gap between ex vivo and in vivo organoids, paving the door for new cancer therapies. With the proper use of these extraordinary 3D cultures, a thorough development of high-throughput drug screening for improved prediction may be possible.
References
- Qian X, Song H, Ming GL. Brain organoids: advances, applications and challenges. Development, 2019; 146: dev166074. doi: 10.1242/dev.166074.
- Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. U.S.A. 2013; 110; 20284–20289. doi: 10.1073/pnas.1315710110
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature, 2013; 501: 373–379. doi: 10.1038/nature12517
- Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, et al. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol 2017; 35: 659–666. doi: 10.1038/nbt.3906.
- Paşca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods, 2015; 12: 671. doi: 10.1038/nmeth.3415.
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell; 2016; 165: 1238–1254. doi: 10.1016/j.cell.2016.04.032.
- Qian X, Jacob F, Song MM, Nguyen HN, Song H, Ming GL. Generation of human brain region–specific organoids using a miniaturized spinning bioreactor. Nat. Protoc, 2018; 13: 565–580. doi: 10.1038/nprot.2017.152.
- Qian X, Song H, Ming GL. Brain organoids: advances, applications and challenges. Development, 2019; 146: dev166074.doi: 10.1242/dev.166074.
- Amin ND, Paşca SP. Building models of brain disorders with three-dimensional organoids, Neuron 2018; 100: 389-405. doi: 10.1016/j.neuron.2018.10.007
- Azzarelli R. Organoid Models of Glioblastoma to Study Brain Tumor Stem Cells. Frontiers in Cell and Developmental Biology, 2020; 8.
- Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science, 2014; 80: 344. 1396–1401. doi: 10.1126/science.1254257.
- Tirosh I, Venteicher AS, Hebert C, Escalante LE, Patel AP, Yizhak K, et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature, 2016; 539, 309–313. doi: 10.1038/nature20123
- Lan X, Jörg DJ, Cavalli FMG, Richards LM, Nguyen LV, Vanner RJ, et al. Fate mapping of human glioblastoma reveals an invariant stem cell hierarchy. Nature, 2017; 549: 227–232. doi: 10.1038/nature23666.
- Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell, 2019; 178: 835–849.e21. doi: 10.1016/j.cell.2019.06.024.
- Vanner RJ, Remke M, Gallo M, Selvadurai HJ, Coutinho F, Lee L, et al. Quiescent Sox2+ cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell, 2014; 26: 33–47. doi: 10.1016/j.ccr.2014.05.005.
- Boj SF, Hwang CI, Baker LA, Chio IIC, Engle DD, Corbo V, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell, 2015; 160: 324–338. doi: 10.1016/j.cell.2014.12.021.
- Van De Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell, 2015; 161: 933–945. doi: 10.1016/j.cell.2015.03.053.
- Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell, 2018; 172: 373–386.e10. doi: 10.1016/j.cell.2017.11.010.
- Yan HHN, Siu HC, Law S, Ho SL, Yue SSK, Tsui WY, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell, 2018; 23: 882–897.e11. doi: 10.1016/j.stem.2018.09.016.
- Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ, Chun SM, et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun, 2019; 10: 3991.
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia, 2002; 39: 193–206. doi: 10.1002/glia.10094.
- Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. U.S.A, 2003; 100: 15178–15183. doi: 10.1073/pnas.2036535100.
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma (Cancer Research (October 2004) 64 (7011-7021). Cancer Res, 2004; 64: 8130.
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature, 2004; 432: 396–401. doi: 10.1038/nature03128.
- Tunici P, Bissola L, Lualdi E, Pollo B, Cajola L, Broggi G, et al. Genetic alterations and in vivo tumorigenicity of neurospheres derived from an adult glioblastoma. Mol. Cancer, 2004; 3: 25. doi: 10.1186/1476-4598-3-25.
- Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene, 2004; 23: 9392–9400. doi: 10.1038/sj.onc.1208311.
- Fael Al-Mayhani TM, Ball SLR, Zhao JW, Fawcett J, Ichimura K, Collins PV, et al. An efficient method for derivation and propagation of glioblastoma cell lines that conserves the molecular profile of their original tumours. J. Neurosci. Methods, 2009; 176: 192–199. doi: 10.1016/j.jneumeth.2008.07.022.
- Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell, 2009; 4: 568–580. doi: 10.1016/j.stem.2009.03.014.
- Vukicevic V, Jauch A, Dinger TC, Gebauer L, Hornich V, Bornstein SR, et al. Genetic instability and diminished differentiation capacity in long-term cultured mouse neurosphere cells. Mech. Ageing Dev, 2010; 131: 124–132. doi: 10.1016/j.mad.2010.01.001.
- Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol, 2005; 3:1594–1606. doi: 10.1371/journal.pbio.0030283.
- Pollard SM, Conti L, Sun Y, Goffredo D, Smith A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb. Cortex, 2006; 16: i112–i120. doi: 10.1093/cercor/bhj167.
- Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell, 2009; 4: 568–580. doi: 10.1016/j.stem.2009.03.014
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma (Cancer Research (October 2004) 64 (7011-7021). Cancer Res, 2004; 64: 8130.
- Azari H, Millette S, Ansari S, Rahman M, Deleyrolle LP, Reynolds BA. Isolation and expansion of human glioblastoma multiforme tumor cells using the neurosphere assay. J. Vis. Exp, 2011; 56: e3633. doi: 10.3791/3633
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science, 1992; 255: 1707–1710. doi: 10.1126/science.1553558.
- Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, et al. A new method for the rapid and long-term growth of human neural precursor cells. J. Neurosci. Methods, 1998; 85: 141–152. doi: 10.1016/s0165-0270(98)00126-5.
- Reynolds BA, Rietze RL. Neural stem cells and neurospheres – Re-evaluating the relationship. Nat. Methods, 2005; 2: 333–336. doi: 10.1038/nmeth758.
- Bian S, Repic M, Guo Z, Kavirayani A, Burkard T, Bagley JA, et al. Genetically engineered cerebral organoids model brain tumor formation. Nat. Methods, 2018; 15: 631–639. doi: 10.1038/s41592-018-0070-7.
- Ogawa J, Pao GM, Shokhirev MN, Verma IM. Glioblastoma model using human cerebral organoids. Cell Rep, 2018; 23: 1220–1229. doi: 10.1016/j.celrep.2018.03.105.
- Hubert CG, Rivera M, Spangler LC, Wu Q, Mack SC, Prager BC, et al. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res, 2016; 76: 2465–2477. doi: 10.1158/0008-5472.can-15-2402.
- Linkous A, Balamatsias D, Snuderl M, Edwards L, Miyaguchi K, Milner T, et al. Modeling patient-derived glioblastoma with cerebral organoids. Cell Rep, 2019; 26: 3203–3211.e5. doi: 10.1016/j.celrep.2019.02.063.
- da Silva B, Mathew RK, Polson ES, Williams J, Wurdak H. Spontaneous glioblastoma spheroid infiltration of early-stage cerebral organoids models brain tumor invasion. SLAS Discov, 2018; 23: 862–868. doi: 10.1177/2472555218764623.
- Plummer S, Wallace S, Ball G, Lloyd R, Schiapparelli P, Quiñones-Hinojosa A, et al. A Human iPSC-derived 3D platform using primary brain cancer cells to study drug development and personalized medicine. Sci. Rep, 2019; 9: 1–11.
- Bhaduri A, Di Lullo E, Jung D, Müller S, Crouch EE, Espinosa CS, et al. Outer radial glia-like cancer stem cells contribute to heterogeneity of glioblastoma. Cell Stem Cell, 2020; 26: 48–63.e6. doi: 10.1016/j.stem.2019.11.015.
- Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CHH, Newman SA, et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron, 2017; 94; 278–293.e9. doi: 10.1016/j.neuron.2017.03.042.
- Brownjohn PW, Smith J, Solanki R, Lohmann E, Houlden H, Hardy J, et al. Functional studies of missense TREM2 mutations in human stem cell-derived microglia. Stem Cell Rep, 2018; 10: 1294–1307. doi: 10.1016/j.stemcr.2018.03.003.
- Ormel PR, Vieira de Sá R, van Bodegraven EJ, Karst H, Harschnitz O, et al. Microglia innately develop within cerebral organoids. Nat. Commun, 2018; 9: 4167.
- Jacob F, Salinas RD, Zhang DY, Rourke DMO. Resource a patient-derived glioblastoma organoid model and resource a patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell, 2020; 180: 188–204.e22. doi: 10.1016/j.cell.2019.11.036.
- Daviaud N, Friedel RH, Zou H. Vascularization and engraftment of transplanted human cerebral organoids in mouse cortex. eNeuro, 2018; 5: 1–18. doi: 10.1523/ENEURO.0219-18.2018.
- Lancaster MA. Brain organoids get vascularized. Nat. Biotechnol, 2018; 36: 407–408. doi: 10.1038/nbt.4133.
- Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, et al. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol, 2018; 36: 432–441. doi: 10.1038/nbt.4127
- Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc, 2014; 9: 2329–2340. doi: 10.1038/nprot.2014.158.
- Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Yang SM, Berger DR, et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature, 2017; 545: 48–53. doi: 10.1038/nature22047.
- Amin ND, Paşca SP. Building models of brain disorders with three-dimensional organoids. Neuron, 2018; 100; 389–405. doi: 10.1016/j.neuron.2018.10.007.
- Qian X, Song H, Ming GL. Brain organoids: advances, applications and challenges. Development, 2019; 146: dev166074. doi: 10.1242/dev.166074.
- Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature, 2019; 570; 523–527. doi: 10.1038/s41586-019-1289-x.
- Orcheston-Findlay L, Bax S, Utama R, Engel M, Govender D, O’Neill G. Advanced Spheroid, Tumouroid and 3D Bioprinted In-Vitro Models of Adult and Paediatric Glioblastoma. International Journal of Molecular Sciences, 2021; 22(6); p.2962.
- Driehuis E, Kolders S, Spelier S, Lõhmussaar K, Willems SM, Devriese LA, de Bree R, de Ruiter EJ, Korving J, Begthel H, et al. Oral Mucosal Organoids as a Potential Platform for Personalized Cancer Therapy. Cancer Discov, 2019a; 9(7): 852–871.
- Driehuis E, Spelier S, Beltrán Hernández I, de Bree R, Willems SM, Clevers H, Oliveira S. Patient-derived head and neck cancer organoids recapitulate egfr expression levels of respective tissues and are responsive to egfr-targeted photodynamic therapy. J Clin Med, 2019b; 8(11): 1880.
- Nanki Y, Chiyoda T, Hirasawa A, Ookubo A, Itoh M, Ueno M, et al. Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing. Scientific Reports, 2020; 10(1).
- Kopper O, et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med, 2019; 25; 838–849.
- Jabs, J. et al. Screening drug effects in patient-derived cancer cells links organoid responses to genome alterations. Mol. Syst. Biol, 2017; 13: 955.
- Neal, J. T. et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell, 2018; 175; 1972-1988.e1916 2018.
- Li X, Francies HE, Secrier M, Perner J, Miremadi A, Galeano-Dalmau N, et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat Commun, 2018; 9(1): 2983. doi: 10.1038/s41467-018-05190-9.
- Derouet MF, Allen J, Wilson GW, Ng C, Radulovich N, Kalimuthu S, et al. Towards personalized induction therapy for esophageal adenocarcinoma: organoids derived from endoscopic biopsy recapitulate the pre-treatment tumor. Sci Rep, 2020; 10(1): 14514. doi: 10.1038/s41598-020-71589-4.
- Gao M, Lin M, Rao M, Thompson H, Hirai K, Choi M, et al. Development of Patient-Derived Gastric Cancer Organoids from Endoscopic Biopsies and Surgical Tissues. Ann Surg Oncol, 2018; 25(9): 2767–2775. doi: 10.1245/s10434-018-6662-8.
- Steele NG, Chakrabarti J, Wang J, Biesiada J, Holokai L, Chang J, et al. An Organoid-Based Preclinical Model of Human Gastric Cancer. Cell Mol Gastroenterol Hepatol, 2019; 7(1): 161–184. doi: 10.1016/j.jcmgh.2018.09.008.
- Li J, Xu H, Zhang L, Song L, Feng D, Peng X, et al. Malignant ascites-derived organoid (MADO) cultures for gastric cancer in vitro modelling and drug screening. J Cancer Res Clin Oncol, 2019; 145(11): 2637–2647. doi: 10.1007/s00432-019-03004-z.
- Arena S, Corti G, Durinikova E, Montone M, Reilly NM, Russo M, et al. A Subset of Colorectal Cancers with Cross-Sensitivity to Olaparib and Oxaliplatin. Clin Cancer Res; 2020; 26(6): 1372–1384. doi: 10.1158/1078-0432.CCR-19-2409.
- Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernandez-Mateos J, Khan K, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science, 2018; 359(6378): 920–926. doi: 10.1126/science.aao2774.
- Ganesh K, Wu C, O’Rourke KP, Szeglin BC, Zheng Y, Sauve CG, et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med, 2019; 25(10): 1607–1614. doi: 10.1038/s41591-019-0584-2.
- Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L, et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell, 2020; 26(1): 17–26. e6. doi: 10.1016/j.stem.2019.10.010.
- Loong HH, Wong AM, Chan DT, Cheung MS, Chow C, Ding X, et al. Patient-derived tumor organoid predicts drugs response in glioblastoma: A step forward in personalized cancer therapy? J Clin Neurosci, 2020; 78: 400–402. doi: 10.1016/j.jocn.2020.04.107.
- Chadwick M, Yang C, Liu L, Gamboa CM, Jara K, Lee H, et al. Rapid Processing and Drug Evaluation in Glioblastoma Patient-Derived Organoid Models with 4D Bioprinted Arrays. iScience, 2020; 23(8): 101365. doi: 10.1016/j.isci.2020.101365.
- Scognamiglio G, De Chiara A, Parafioriti A, Armiraglio E, Fazioli F, Gallo M, et al. Patient-derived organoids as a potential model to predict response to PD-1/PD-L1 checkpoint inhibitors. Br J Cancer (2019) 121(11):979–82. doi: 10.1038/s41416-019-0616-1.
- Saengwimol D, Rojanaporn D, Chaitankar V, Chittavanich P, Aroonroch R, Boontawon T, et al. A three-dimensional organoid model recapitulates tumorigenic aspects and drug responses of advanced human retinoblastoma. Sci Rep, 2018; 8(1): 15664. doi: 10.1038/s41598-018-34037-y.
- Sartore RC, Cardoso SC, Lages YV, Paraguassu JM, Stelling MP, et al. 2017. Trace elements during primordial plexiform network formation in human cerebral organoids. PeerJ, 2017; 5: e2927.
- Fiore D, Ramesh P, Proto MC, Piscopo C, Franceschelli S, Anzelmo S, et al. Rimonabant Kills Colon Cancer Stem Cells without Inducing Toxicity in Normal Colon Organoids. Front. Pharmacol, 2018; 8: 949. doi: 10.3389/fphar.2017.00949.
- Lu W, Rettenmeier E, Paszek M, Yueh M-F, Tukey RH, Trottier J, et al. Crypt Organoid Culture as an in Vitro Model in Drug Metabolism and Cytotoxicity Studies. Drug Metab. Dispos, 2017; 45: 748–754. doi: 10.1124/dmd.117.075945.
- Pamies D, Block K, Lau P, Gribaldo L, Pardo CA, Barreras P, et al. Rotenone exerts developmental neurotoxicity in a human brain spheroid model. Toxicol. Appl. Pharmacol, 2018; 354: 101–114. doi: 10.1016/j.taap.2018.02.003.
- Chhibber T, Bagchi S, Lahooti B, Verma A, Al-Ahmad A, Paul MK, et al. CNS organoids: An innovative tool for neurological disease modeling and drug neurotoxicity screening. Drug Discov. Today, 2019; 25: 456–465. doi: 10.1016/j.drudis.2019.11.010.
- Schielke C, Hartel C, Durante M, Ritter S, Schroeder IS. Solving the Issue of Ionizing Radiation Induced Neurotoxicity by Using Novel Cell Models and State of the Art Accelerator Facilities. Front. Phys, 2020; 8: 417. doi: 10.3389/fphy.2020.568027.
- Liu F, Huang J, Liu Z. Vincristine Impairs Microtubules and Causes Neurotoxicity in Cerebral Organoids. Neuroscience, 2019; 404: 530–540. doi: 10.1016/j.neuroscience.2018.12.047.

