Diagnosis of Hepatopulmonary Syndrome Following Microwave Ablation of Hepatocellular Carcinoma
Lisa Barrett, Kumar Sarvottam, Richard Kalman, Sadia Benzaquen, Ena Gupta
Department of Internal Medicine, Albert Einstein Medical Center, Philadelphia
Department of Pulmonary & Critical Care, Albert Einstein Medical Center, Philadelphia
Department of Gastroenterology, Albert Einstein Medical Center, Philadelphia
Received Date: 28/02/2022; Published Date: 11/03/2022
*Corresponding author: Lisa Barrett, DO, Department of Internal Medicine, Einstein Medical Center, 5501 Old York Road, Philadelphia, PA 19141
Abstract
Microwave Ablation (MWA) is one of the definitive treatment modalities for Hepatocellular Carcinoma (HCC). Although MWA is associated with a favorable safety profile, major complications may arise immediately post procedure, requiring hospitalization and urgent treatment. This case report discusses Hepatopulmonary Syndrome (HPS) in an acutely decompensated patient status post MWA who ultimately required urgent liver transplant.
Keywords: Microwave ablation; Cirrhosis; Hepatocellular carcinoma; Hepatopulmonary syndrome
Introduction
Microwave Ablation (MWA) is one of the definitive treatment modalities for Hepatocellular Carcinoma (HCC). It is generally believed that MWA is a low-risk procedure, however complications may arise. We diagnosed Hepatopulmonary Syndrome (HPS) following a MWA in an acutely decompensated patient which ultimately required urgent liver transplantation. Our case report cautions timely identification and appropriate management of HPS due to its high mortality and the possible complication.
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer deaths worldwide [1]. Radiofrequency ablation (RFA) and microwave ablation (MWA) are the common therapeutic modalities utilized to treat small HCCs. MWA uses an electromagnetic field to inflict thermal injury on the tumor and ultimately cause coagulation necrosis [2]. Although MWA is associated with a favorable safety profile, a major complication rate of 2.6-4.6% has been reported [3]. Common complications of MWA or RFA include intraperitoneal bleeding, intrahepatic hematoma, bile leak, bile duct injury [4]. Pulmonary complications are rare and can include pleural effusion, hemothorax or diaphragmatic hernia [4]. Further liver decompensation manifesting as hepatopulmonary syndrome (HPS) has rarely been reported as a complication after MWA [5]. There is one reported case of HPS following RFA [3]. We report a case of a middle-aged man with liver cirrhosis and HCC who developed acute liver decompensation and was subsequently diagnosed with HPS following MWA.
Clinical Vignette
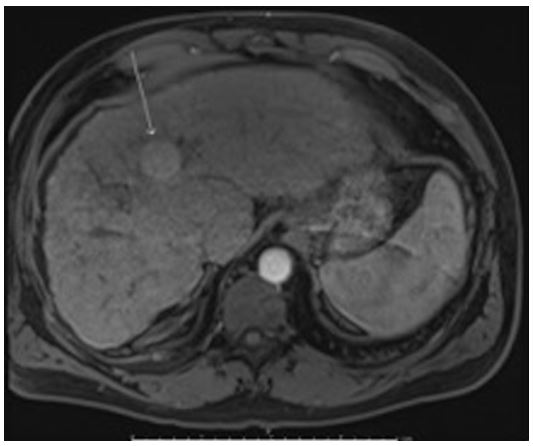
A 57-year-old male with a past medical history of Factor V Leiden heterozygosity with left leg deep venous thrombosis, diabetes mellitus type 2, hypertension, and hepatitis C cirrhosis complicated by esophageal varices, hepatic encephalopathy, and a 2.4 cm HCC was admitted to the hepatology service with acute onset shortness of breath and altered mentation following MWA. Of note, he was previously treated for hepatitis C with sofosbuvir/velpatasvir and ribavirin with sustained virologic response. On magnetic resonance imaging he was found to have a 2.8cm X 2.7cm HCC in segment 4A (Figure 1). MWA was performed at 65W for 10 minutes for the observed HCC. Hypoxia developed post procedure and he was sent to the emergency department. On admission he was saturating 91% on 4L oxygen (O2) supplementation via nasal canulae (NC). Physical exam was significant for orthodeoxia with oxygen saturation of 92%, 88% and 84% on 4L NC in the supine, sitting and standing positions respectively. He was disoriented and was also noted to have asterixis. Laboratory values demonstrated sodium 134, creatinine 0.7, total bilirubin 7.5, INR 1.5, white blood cell count 6.4 and Model for End-Stage Liver Disease (MELD) score of 20. MELD score one month prior to admission was 17.
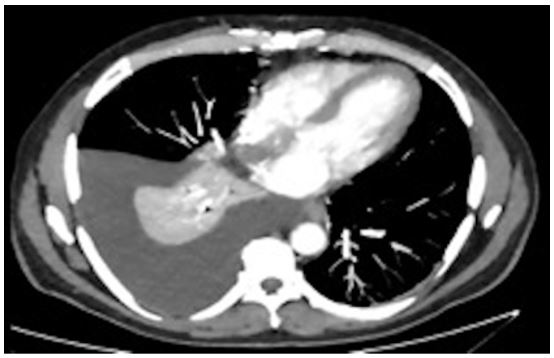
Chest x-ray and Computed Tomography (CT) scan showed diffuse opacities bilaterally concerning for pulmonary edema and right sided pleural effusion (Figure 2). He had no prior history of pleural effusions. He underwent a bedside thoracentesis with the removal of 1.8 L of transudative fluid consistent with hepatic hydrothorax. The patient’s edema resolved with diuresis. He continued to require 4L O2 supplementation via NC.
Echocardiogram prior to hospitalization had shown normal left and right ventricular systolic function and Patent Foramen Ovale (PFO) with evidence of intra-cardiac shunt. The patient underwent right and left heart catheterization showing normal right sided pressures. Pulmonary Artery (PA) pressure was 14/5 mmHg with a mean PA pressure of 7 mmHg. A normal Pulmonary Capillary Wedge Pressure (PCWP) of 3 mmHg and normal peripheral vascular resistance (PVR) of 0.34 woods unit. Shunt study showed no left to right shunting.
On day 6 of hospitalization, a Transesophageal Echocardiogram (TEE) was performed to better evaluate intracardiac shunt. TEE showed a fenestrated atrial septal aneurysm with a small right to left shunt through a small PFO, and a much larger intrapulmonary shunt demonstrating abundant late appearing bubbles appearing in the left atrium. Arterial blood gas on room air was 7.47/35/48 with a calculated A-a gradient of 48.0 mmHg. Arterial blood gas on 100% Fio2 was 7.45/37/119 with a shunt fraction calculated to be 30%. Based on the above findings of late bubbles on bubble study and elevated shunt fraction, hypoxia was attributed predominantly to HPS. Additional workup for hypoxia included CT pulmonary arteriogram, which was negative for pulmonary embolism and demonstrated chronic peripheral reticulation with lower lobe predominance unchanged from 5 months prior. Of note, pre-hospital pulmonary function testing showed normal spirometry with an isolated reduction in diffusing capacity for carbon monoxide (DLCO) of 54%.
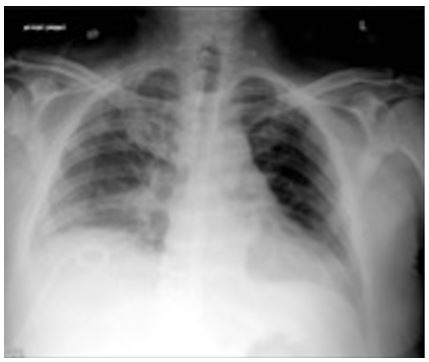
MELD exception points were obtained for the patient’s HPS diagnosis and he was transplanted on day 26 of hospitalization. He was later discharged to inpatient rehabilitation on room air. Chest X-ray on postoperative day 10 (Figure 3) shows no pleural effusion.
The patient is currently one year post transplant. He remains on room air, has not experienced rejection or post-operative complications.
Discussion
Although MWA for liver tumors is usually safe, pulmonary complications such as pneumothorax and pleural effusion have been noted, especially when the tumor is in close proximity to the diaphragm [6]. However, liver decompensation following MWA is extremely rare. Our patient developed worsening liver failure complicated by hepatic encephalopathy, hepatic hydrothorax and was also diagnosed with HPS following MWA. His encephalopathy improved with treatment however hepatic hydrothorax and hypoxia continued to worsen throughout his hospital course. Hepatopulmonary syndrome was also challenging to diagnose due to presence of PFO and some small intra cardiac shunting. However, presence of orthodeoxia, late bubbles after agitated saline injection and elevated shunt fraction were helpful in the diagnosis of HPS.
Respiratory complications following MWA are normally transient and resolve after symptomatic management, however worsening liver failure may not be reversible [7]. Acute HPS is a rare complication and has only been reported once in the past following RFA [3]. HPS is the result of pulmonary microvascular dilation and angiogenesis caused by decreased clearance of vasodilators, such as nitric oxide, produced as a result of liver necrosis [6]. The acute necrosis of liver tissue caused by the MWA procedure presumably leads to hepatic decompensation in a cirrhotic patient. Complications following MWA have been associated with higher number of insertions, number of tumors, and larger lesions [8]. Advanced underlying liver disease with poor reserve or multiple ablations within a short period of time have also been noted to have consequent hepatic decompensation [9]. However, risk factors for liver decompensation post MWA or RFA are not well studied.
The presence of HPS markedly increases mortality in the setting of cirrhosis [9]. Along with supportive treatment, liver transplantation is the only curative treatment [10]. Vasoconstrictors have not been found to be efficacious for HPS and transplant outcomes are worse when patients undergo transplant with more advanced HPS [11,12]. It is essential to recognize this condition early and expedite evaluation for liver transplant. More studies are needed to understand risk factors predisposing to this syndrome and to determine the incidence of HPS as a result of MWA or RFA.
Conflict of Interest: No conflict of interest for any of the authors
Declaration of interests: No conflict of interest
Role of Funding Source: No role of funding
Prior presentation: October 2020, American College of Gastroenterology Annual Scientific Meeting, USA
Author contributions: All authors contributed equally to the manuscript
References:
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer. Wiley-Liss Inc., 2019; 144: p. 1941–1953.
- Poulou LS, Botsa E, Thanou I, Ziakas PD, et al. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World Journal of Hepatology. Baishideng Publishing Group Co, 2015; 7: p. 1054–1063.
- Wang Y, Ma K, Zhong A, Xiong Q, et al. Hepatopulmonary syndrome after radiofrequency ablation of recurrent intrahepatic cholangiocarcinoma: A case report. Onco Targets Ther, 2019; 12: 2431–2438.
- Rhim H. Complications of radiofrequency ablation in hepatocellular carcinoma. Vol. 30, Abdominal Imaging, 2005; 30: p. 409–418.
- Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol, 2015; 7(8): 1054-1063. doi:10.4254/wjh.v7.i8.1054.
- Zhang J, Fallon MB. Hepatopulmonary syndrome: Update on pathogenesis and clinical features [Internet]. Nature Reviews Gastroenterology and Hepatology. 2012; 9: p. 539–549.
- Abbott DE, Sohn VY, Hanseman D, et al. Cost-effectiveness of simultaneous resection and RFA versus 2-stage hepatectomy for bilobar colorectal liver metastases. J Surg Oncol [Internet], 2014;109(6): 516–520.
- Livraghi T, Meloni F, Solbiati L, et al. Complications of microwave ablation for liver tumors: Results of a multicenter study. Cardiovasc Intervent Radiol [Internet], 2012; 35(4): 868–874.
- Kong WT, Zhang WW, Qiu YD, et al. Major complications after radiofrequency ablation for liver tumors: Analysis of 255 patients. World J Gastroenterol. 2009; 15(21): 2651.
- Auzinger G, Willars C, Loveridge R, et al. Extracorporeal membrane oxygenation for refractory hypoxemia after liver transplantation in severe hepatopulmonary syndrome: A solution with pitfalls. Liver Transplant [Internet]. 2014; 20(9): 1141–1144.
- Krowka MJ, Dickson ER, Cortese DA. Hepatopulmonary syndrome: Clinical observations and lack of therapeutic response to somatostatin analogue. Chest [Internet], 1993; 104(2): 515–521.
- Goldberg DS, Krok K, Batra S, et al. Impact of the hepatopulmonary syndrome MELD exception policy on outcomes of patients after liver transplantation: An analysis of the UNOS database. Gastroenterology, 2014; 146(5): 1256-1265.e1.

