Analysis of Bio-actives from Cyperus Rotundus L. On Obesity Based Inflammatory Molecular Targets: In-Silico Study
Nikhil Pandey, Priya Shree, Priyanka Mishra, Yamini B Tripathi*
Department of Medicinal Chemistry, Institute of Medical Sciences, Banaras Hindu University, India
Received Date: 16/02/2022; Published Date: 07/03/2022
*Corresponding author: Prof. Yamini B Tripathi, Department of Medicinal Chemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, India
Abstract
In this contemporary work we have analyzed the natural compounds present in Cyperus rotundus L. roots which could help in reduction in obesity through downregulation of pro-inflammatory cytokines. As these pro-inflammatory are higher in obese patients and thus initiate a prolonged chronic inflammation condition. Hence to inhibit or downregulation of COX-2, TNF-alpha, NFkB and IL-6 can attenuate the inflammation condition resulting in decrease in inflammation status which could aid in the management if obesity condition. As currently modern drugs come with side effects therefore search of natural products is need of the time. Thus, Cyperus rotundus already known to be hypolipidemic in nature, could also inhibit the inflammation status in the obesity. Bioactive from Cyperus rotundus L. could inhibit the TNF-alpha, NFkB, IL-6 and COX-2 pathway. Hence through the in-silico analysis through molecular docking we are trying to study the interaction of these bioactive against these proteins.
Introduction
Obesity, a modern-day epidemic with alarmingly high rates, is related with comorbidities such as cardiovascular disease, arthritis, certain malignancies, and degenerative illnesses of the brain and other organs [1]. Obesity, in particular, is a primary cause of insulin resistance and type 2 diabetes. Inflammation is one of the important processes connected with the development of insulin resistance, diabetes, and related disorders, as accumulating data has indicated over the last decade, and obesity is now regarded a condition of chronic low-grade inflammation [2]. Aside from its traditional role as an energy storage depot, adipose tissue is a key endocrine organ that secretes a variety of hormones, the levels of which are regulated by the degree of adiposity [3]. Obesity causes an increase in pro-inflammatory cytokine levels, which aids in the advancement of this morbid illness. Under morbid obesity situations, the COX-2 gene and immunoreactive proteins have been shown to be significantly expressed and upregulated in adipose tissue (AT) [4]. Similarly, TNF-alpha, a proinflammatory cytokine, shown to be overexpressed in the adipose tissue of obese mice [5]. TLRs are well-studied immunological receptors that promote inflammation. Obesity and MetS are both characterised by low-grade chronic inflammation, which may be induced by TLR2 and TLR4 activation [6]. Presence of plant’s phytochemical provides a perspective to modulate the level of such pro-inflammatory cytokines in order to manage the obesity condition. Here through the application of In-silico analysis, presence of certain phytochemicals and their interaction with these target receptors was analysed.
Target and their rational
NFκB- It has been proposed that consuming HFD may cause low-grade inflammation in adipose tissue. NF-B activity is increased in mice fed a high-fat diet.The adipose tissue in the abdomen area, in particular, has been related to the development of illnesses such as type 2 diabetes and atherosclerosis. HFD and obesity generally activate NF-B by a factor of two, which is substantially lower than the 10–100-fold activation seen in acute inflammatory responses. This supports the idea of obesity as a persistent low-grade inflammatory disorder.NF-B activity is increased in mice fed a high-fat diet [7].
TLR4- The toll-like receptor 4 (TLR4) signalling pathway has been identified as one of the primary initiators of the obesity-induced inflammatory response. TLRs are well-studied immunological receptors that promote inflammation. TLRs are activated and expressed when pathogen-associated molecular patterns and endogenous (host-derived) ligands produced by distinct cell types are recognised. TLRs, particularly TLR2 and TLR4, cause insulin resistance, which plays an important role in the pathophysiology of obesity and MetS. Obesity and MetS are both characterised by low-grade chronic inflammation, which may be induced by TLR2 and TLR4 activation. TLRs, particularly TLR4, are activated by fatty acids and endotoxinemia (a measure of gut permeability), both of which are associated with obesity and MetS, resulting in nuclear factor-B activation and increased release of inflammatory biomediators [8].
TNF-α- In the obese condition, adipocytes produce and release TNF-a,when compared to lean animals, the mRNA and protein levels are higher per unit RNA, protein, or cell [9]. Furthermore, obese people have more adipose tissue, which frequently contains more fat cells, which would be predicted to have an extra amplifying influence on total body TNF-a production. TNF-A is produced and released by adipose tissue, with its levels correlated with obesity and insulin resistance. TNF- and/or its receptors targeting has been proposed as a viable therapy for insulin resistance and type 2 diabetes [10].
COX-2- Rats on a High-Fat Diet (HFD) were found to have higher levels of COX-1 and COX-2 expression. COX inhibition has been linked to reduced production of pro-inflammatory cytokines and chemokines such as tumour necrosis factor (Tnfa) and monocyte chemotactic protein-1 (Ccl2) [11]. Under morbid obesity situations, the COX-2 gene and immunoreactive proteins have been shown to be significantly expressed and upregulated in adipose tissue (AT) [12]. Environmental stress-induced COX-2 expression, on the other hand, has been shown to play distinct roles under different pathological and physiological conditions; for example, over-expression of the COX-2 gene in white AT (WAT) has been shown to induce de novo brown AT (BAT) recruitment in WAT and then facilitate systemic energy expenditure to protect mice against high-fat diet-induced obesity [13].
Hence the use of molecular docking tool to establish the overall role of Cyperus rotundus on the molecular target of obesity’s centred inflammatory molecules will give an insight how these bio actives could bond to proteins of interest. Cyperus rotundus L. was searched at Indian Medicinal Plants, Phytochemistry and Therapeutics (IMPPAT) a database curated by CSIR for their respective phytoconstituent. The structure of these were then retrieved from PubChem database and were saved in 2D-SDF format. The therapeutic target was searched from literature review and the protein structure of these target were retrieved from protein data bank from RCSB database and were rendered as target by the use of Discovery studio software. Finally, with the use of YASARA which is an auto dock based molecular docking tool for analysing the binding energy and interaction of ligand and target.
Materials and Methods
Protein preparation and active site prediction
RCSB Protein Data Bank (https://www.rcsb.org/) was used to retrieve 3D crystal structures NFKB, TLR-4, TNF-α and COX-2 having PDB ID: 1NFKB, 3FXI, 2AZ5 and 6BL4 respectively. Further the “Prepare protein” protocol of BIOVIA Discovery studio 4.5 (DS 4.5) was used for preparation of proteins at physiological pH 7.4. Water molecules and other heteroatoms were removed from the crystal structures.
Ligands Selection
IMPPAT-Indian Medicinal Plants Phytochemistry And Therapeutics and Dr. Duke’s Phytochemical and Ethnobotanical databases and literature search were used for retrieval of bioactive phytochemicals structures were taken from PubChem database (https://pubchem.ncbi.nlm.nih.gov/) in 2D SDF format. Geometry minimization and conversion of all retrieved small molecules in 3D PDB format was done with the help of DS 4.5.
Molecular docking
Autodock Vina based YASARA software has been used to perform molecular docking. Selected phytochemicals IMMPAT were docked with NFKB, TLR-4, TNF-α and COX-2 proteins. For molecular docking study in YASARA, prepared receptor and ligand files were used to set target and opt play macro option to run the job. Macro file dockrun mcr option in YASARA was used for the calculation of interaction energy between the receptor and selected ligands independently. 25 VINA docking runs of the ligand object 2 to the receptor object 1 was done with the help of YASARA. Further docked complexes were saved in PDB file format for 2D-3D interactive visualisation study with the help of D.S (4.5). The result log files were sorted based on binding energy [kcal/mol] and dissociation constant [pM]. The compounds with more positive binding energies indicate stronger binding, and negative energies indicate no binding.
Result
Molecular docking calculation
Molecular docking study revealed 4 different active phytochemicals out of 40 from…. showing significant binding affinity with NFKB, TLR-4, TNF-α and COX-2 proteins. Table 1 shows the list of the active phytochemicals which showed significant binding affinity (≥7.5 kcal/mole) with NFKB, TLR-4, TNF-α and COX-2 protein.
Table 1: List of phytochemicals with binding energy and dissociation constant (pM) ≥7.5 kcal/mol for NFKB, TLR-4, TNF-α and COX-2 proteins.
Inhibitor for NFκB
Based on YASARA scoring molecular docking study revealed that out of 40 active phytochemicals of CRR ,one phytochemical namely Grossamide showed significant binding affinity with NFκB. Grossamide was found to have maximum binding energy of 7.78 kcal/mol. It forms various 2D-3D interaction including conventional and carbon hydrogen bonding with the residues Asn 247, Leu 248, Thr 339, Lys 49 and 52. π-alkyl interactions with the residues Arg 51, Ala 135 and Lys 77. Remaining residues showed few Van der Waals interactions (Fig 1a, b).

Figure 1: (a) 3D interaction diagram of Grossamide with NFκB (b) 2D interaction diagram of Grossamide with NFκB.
Inhibitor for TLR-4
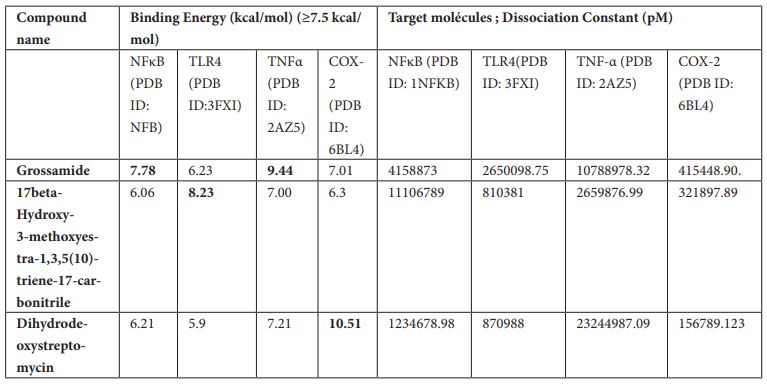
17beta-Hydroxy-3-methoxyestra-1,3,5 (10)-triene-17-carbonitrile was found to inhibit TLR-4 with binding energy 8.23 kcal/mol. Different ligand-protein interactions was formed by 17beta-Hydroxy-3-methoxyestra-1,3,5 (10)-triene-17-carbonitrile which includes conventional hydrogen bonding with the residue’s Ser 534 and Lys 560. Alkyl and π-alkyl interactions with the residues Phe 532 and Leu 511. π-π-stacked interaction was formed by residue Phe 487. Few van der Waals interactions were formed by remaining residues (Figure 2a, b).
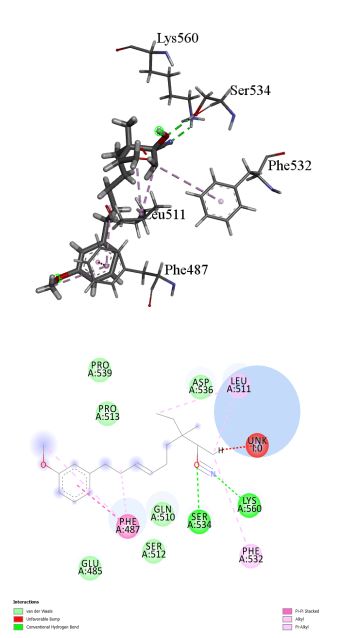
Figure 2: (a) 3D interaction diagram of 17beta-Hydroxy-3-methoxyestra-1,3,5 (10)-triene-17-carbonitrile with TLR-4. (b) 2D interaction diagram of 17beta-Hydroxy-3-methoxyestra-1,3,5 (10)-triene-17-carbonitrile with TLR-4.
Inhibitor for TNF-alpha
Grossamide was found to inhibit TNF-alpha with binding energy 9.44 kcal/mol. Different interactions formed by Grossamide which includes conventional and carbon hydrogen bonding with the residues Phe 64, Gly 121 and Tyr 151, alkyl and π-alkyl interactions was formed with the residues Pro 117, Tyr 119, Tyr 59 and Leu 55. π-π-stacked and π-π-T-shaped was formed by Tyr 119, π-sigma was formed with Leu 63. Many van der Waals interactions formed by remaining residues (Figure 3a, b).

Figure 3: (a) 3D interaction diagram of Grossamide with TNF-alpha (b) 2D interaction diagram of Grossamide with TNF-alpha.
Inhibitor for COX-2
Dihydrodeoxystreptomycin was found to inhibit COX-2 with binding energy 10.51 kcal/mol. Interactions shown by Dihydrodeoxystreptomycin comprises conventional and carbon hydrogen bonds with residues Gly 45, Cys 41, Cys 36, Gly 135, and Asn 34, and Gln 110, alkyl interaction with Met 48. Numerous Van der Waals interactions showed by remaining residues (Figure 4a, b).
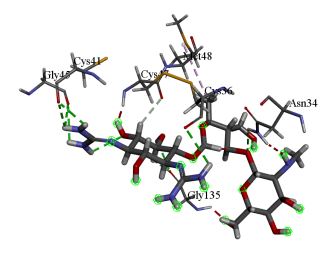
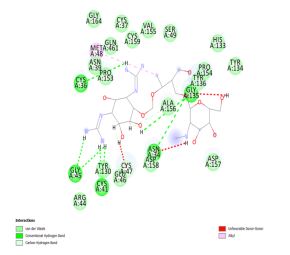
Figure 4: (a) 3D interaction diagram of Dihydrodeoxystreptomycin with COX-2 (b) 2D interaction diagram of Dihydrodeoxystreptomycin with COX-2.
Conclusion
Through this molecular docking study we found that the phytoconstituents from Cyperus rotundus L. roots showed significant binding interaction with targets involved for pathogenesis of chronic inflammation which make obesity condition more severe through disbalancing the Metabolic framework causing the metabolic syndrome .Here the phytoconstituent namely Grossamide showed significant binding affinity with NFκB with the 7.78kcal/mol of binding energy .Indicating its inhibitory activity against NFκB . Presence of 17beta-Hydroxy-3-methoxyestra-1,3,5(10)-triene-17-carbonitrile showed its inhibitory activity with TLR-4 with the binding energy of 8.23 kcal/mol. Similarly, another compound Dihydrodeoxystreptomycin was found to inhibit COX-2 with binding energy 10.51 kcal/mol [14-16]. This suggests that Cyperus rotundus L. could potentially act as Phyto steroidal anti-inflammatory agent and would be beneficial in management of obesity via reducing the inflammation to arrest further progression of Obesity.
References:
- Chakravarthy MV, Joyner MJ and Booth FW. February. An obligation for primary care physicians to prescribe physical activity to sedentary patients to reduce the risk of chronic health conditions. In Mayo clinic proceedings, 2002; 77(2): pp. 165-173.
- Van Greevenbroek MM, Schalkwijk CG, Stehouwer CD. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Neth J Med, 2013; 71(4), pp.174-87.
- Trayhurn P, Bing C, Wood IS. Adipose tissue and adipokines—energy regulation from the human perspective. The Journal of nutrition, 2006; 136(7): pp.1935S-1939S.
- Shelton RC, Miller AH. Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Progress in neurobiology, 2010; 91(4): pp.275-299.
- Lacerda DR, Costa KA, Silveira ALM, Rodrigues DF, Silva AN, Sabino JL, et al. Role of adipose tissue inflammation in fat pad loss induced by fasting in lean and mildly obese mice. The Journal of Nutritional Biochemistry, 2019; 72: p.108208.
- Joseph SV, Edirisinghe I, Burton-Freeman BM. Fruit polyphenols: A review of anti-inflammatory effects in humans. Critical reviews in food science and nutrition, 2016; 56(3): pp.419-444.
- Polavarapu R, Spitz DR, Sim JE, Follansbee MH, Oberley LW, Rahemtulla A, et al. Increased lipid peroxidation and impaired antioxidant enzyme function is associated with pathological liver injury in experimental alcoholic liver disease in rats fed diets high in corn oil and fish oil. Hepatology, 1998; 27(5): pp.1317-1323.
- Rocha DM, Caldas AP, Oliveira LL, Bressan J, Hermsdorff HH. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis, 2016; 244: pp. 211-215.
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of clinical investigation, 1995; 95(5): pp.2409-2415.
- Hube F, Hauner H. The role of TNF-α in human adipose tissue: prevention of weight gain at the expense of insulin resistance? Hormone and metabolic research, 1999; 31(12): pp. 626-631.
- Chen S, Jiang H, Wu X, Fang J. Therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediators of Inflammation, 2016.
- Chan PC, Liao MT, Hsieh PS. The dualistic effect of COX-2-mediated signaling in obesity and insulin resistance. International Journal of Molecular Sciences, 2019; 20(13): p.3115.
- Le Lay S, Simard G, Martinez MC, Andriantsitohaina R. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxidative medicine and cellular longevity, 2014.
- Carlsen H, Haugen F, Zadelaar S, Kleemann R, Kooistra T, Drevon CA, et al. Diet-induced obesity increases NF-κB signaling in reporter mice. Genes & nutrition, 2009; 4(3): pp. 215-222.
- Jialal I, Kaur H, Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. The Journal of Clinical Endocrinology & Metabolism, 2014; 99(1): pp. 39-48.
- Banhos Danneskiold-Samsøe N, Sonne SB, Larsen JM, Hansen AN, Fjære E, Isidor MS, et al. Overexpression of cyclooxygenase-2 in adipocytes reduces fat accumulation in inguinal white adipose tissue and hepatic steatosis in high-fat fed mice. Scientific reports, 2019; 9(1), pp.1-13.

