Will Digital Foot Printing Change the Future of Transfusion Medicine?
Cees Th. Smit Sibinga*
University of Groningen and IQM Consulting, The Netherlands
Received Date: 12/01/2022; Published Date: 18/01/2022
DOI: 10.46998/IJCMCR.2022.17.000412
*Corresponding author: Prof. Cees Th. Smit Sibinga, MD, PhD, FRCP Edin, FRCPath, IQM Consulting, The Netherlands
Abstract
In the day-to-day routine of Transfusion Medicine (TM) large amounts of data are collected and processed in a standardized way and depending on the economy of scale practiced. Blood and its components are not without risk when transfused. That burdens the decision-making and selection for transfusion. The field presents a promising challenge for the introduction of digital foot printing through artificial intelligence (AI) applying deep learning (DL), and machine learning (ML) to improve the quality of outcomes – safety and clinical efficacy of transfusion practice, mitigating unnecessary patient harm.
Keywords: Digital foot printing; Artificial intelligence; Transfusion medicine; Machine learning; Deep learning; Vein-to-vein chain
Introduction
How far have we come?
Times keep changing and so does the world – people, culture, environment, climate, science, technology, communication, standards of healthcare and opportunities. Following the sequential epochs over the past centuries from the dark ages into the enlightenment with its industrialization and socialization, and the dawn of human rights and equality, the world agreed in 1948 on a series of essential and universal human rights documented as a fundament for behaviour, communication, respect, and dignity in a peaceful and nurturing environment for generations to come [1]. Indeed, much has changed to the benefit of mankind. To be able to measure differences and progress in development UN through its development programme UNDP designed in 1990 a series of key indicators [2] to measure a state of development of societies/countries in the ability to –
1) lead a long and healthy life, measured by life expectancy at birth;
2) acquire knowledge, measured by mean years of schooling and expected
years of schooling (primary, secondary and tertiary);
3) achieve a decent standard of living, measured by gross national income per
capita.
These three compose the Human Development Index (HDI). To measure human development more comprehensively, the UNDP presented in 2010 three other composite indices based on protracted inequality. This Inequality-adjusted HDI discounts the HDI according to the extent of inequality [3]
- the Multidimensional Poverty Index (MPI), measuring non-income
dimensions of poverty.
- b) the Inequality-adjusted Human Development Index (IHDI).
- c) the Gender Inequality Index (GII) highlighting the empowerment of
women.
In 2014 in the wake of the UN Sustainable Development Goals 2016-2030 [4], the Gender Development Index (GDI) was added, comparing female and male HDI values in the light of achieving global gender equality [3].
Human development is about human and humanitarian freedoms, about building comprehensively human capacities; not just for a few, not even for most but for ALL. With these indices and indicators of human development a more detailed and comprehensive picture can be drawn of the differences and disparities that still exist. Since the launch of the first Human Development Report in 1990, new challenges - including the current pandemic - to human development, especially inequality and sustainability, require concerted measurement and analytical attention. Data availability is steadily expanding with new opportunities for measurement innovation and disaggregation, and possibilities to improve on quality and storage of data. Technologies are introducing new ways of communicating data- and evidence-based key messages.
These are all opportunities to support development and strengthen the analysis, insight, relevance and reach of the future of Transfusion Medicine, both the manufacturing or procurement part through the principles of economy of scale, and most important the availability and justification for clinical application of safe and efficacious blood and blood components through evidence-based indication-setting and decision-making.
Digital Footprint Within Transfusion Medicine
A vein-to-vein digital footprint with an internal automation network is needed to ensure both blood safety and availability [5]. Acceptance of a blood availability and safety digital footprint depends on an integrated healthcare system and governance, and an open architecture based on common international standards of data elements for all essential vein-to-vein processes within the blood supply chain and to its final administration to the patient.
A vein-to-vein digital footprint within TM will require primary and supportive processes that may be facilitated by Artificial Intelligence (AI). This includes donor motivation, call-up and selection as well as data obtained during the collection process. Checks and balances among expected needs and supplies as well as automation and robotic elements for greater efficiency are envisioned in blood separation, quality testing, quarantine release and final labelling, storage and distribution (cold chain logistics). Management of the blood product, its labelling, and the blood samples for testing could be improved through Radio Frequency Identification (RFID). Automating current manual processes with minimal manual touch is envisioned with RFID e.g., the digital footprint using automation and RFID to direct quality control test tubes for centrifugation or collected whole blood delivered to a centrifuge and blood separator. The digital footprint could even predict and streamline blood product ordering and establish logistics for inventory and delivery efficiency, ensuring the right blood at the right time to the correct location, all while reducing technical manpower and human errors (Figure 1).
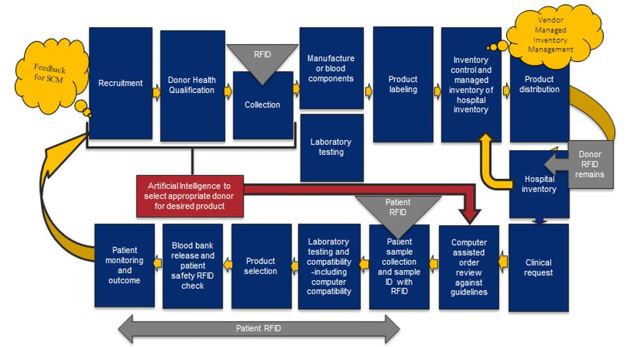
Figure 1: Flow of a potential digital footprint of the integration of RFID into the vein-to-vein transfusion chain. (J. Holmberg, 2014; ref. 5)
The Vein-To-Vein Blood Supply and Transfusion Chain
The manufacturing establishment has two vein-to-vein interfaces – hospitals (clinical consumption, customers), and community (source material, suppliers) (Figure 1c). Both interfaces have a market and communication function. They are elements of a larger environment and form the interconnections with the blood supply system as an integral part of the healthcare system and structure. That determines the stream of information and data, quantitatively and qualitatively. In short, the need is determined by the bedside leading to a demand, which is responded with the supply of products provided by the manufacturing or procurement establishment. The source material human blood comes from the community as a blood market. It operates like a tidal movement.
The blood transfusion chain has two distinctly different parts [6] (Figure 2b).
1) the clinical or consumption part with three major processes
- a) Bedside: diagnosis, indication and ordering.
- b) Laboratory/blood transfusion service: immunohematology (ABO/RhD), selection of blood component and compatibility testing.
- c) Bedside: patient identification, matching with the prepared blood component, transfusion, and observation of outcome.
2) the second part is the collection and manufacturing of the source material – human blood, with four major processes (Figure 2c)
- a) Community: awareness, donor selection and blood collection.
- b) processing: separation of the source material into its cellular components and plasma.
- c) Laboratory: testing of each collected unit for blood group and Transfusion Transmissible Infections (TTI), product specifications.
- d) Storage and distribution: quarantine release and labelling, inventory management, cold chain for storage and transport/distribution to the hospitals.
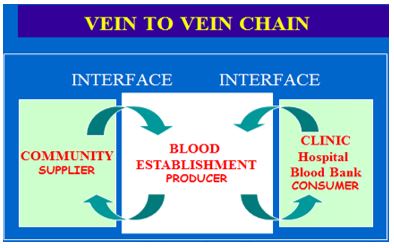
Figure 2a: Vein-to-vein transfusion chain with the 2 interfaces - community and clinical.
The processes of the blood supply
Each of these primary processes has some subprocesses and procedures to transform an input into an output. The primary processes are supported by a series of secondary or supportive processes such as human resource, finance and administration, quality management, education, purchase of consumables and equipment, information and communication technology (ICT), public awareness campaigning, emergency preparedness, waste management, maintenance and repair, domestic services, etc. The supportive processes are elementary to the primary process operations and decisive for the implementation of the strategies initiated by the third layer – steering processes. The steering processes are based on the mission and vision statement or policies of the hospital and blood establishment [7]. Key in this chain or flow is data management – interrelated and interconnected documentation and archiving to achieve consistency, statistical evaluation of outcomes, benchmarking and prediction of volume and changes in each of these processes and procedures. None of these processes is stand-alone, they all are interconnected, forming a complex network of data and information from patient treatment outcome to community awareness, motivation and mobilization of potential donors.
Like in many other businesses and operations it is characterized by plasticity, driven in its flexibility by customer needs and demands, a vein-to-vein flow of processes and procedures that for their consistency depend on documentation, competency and stewardship.
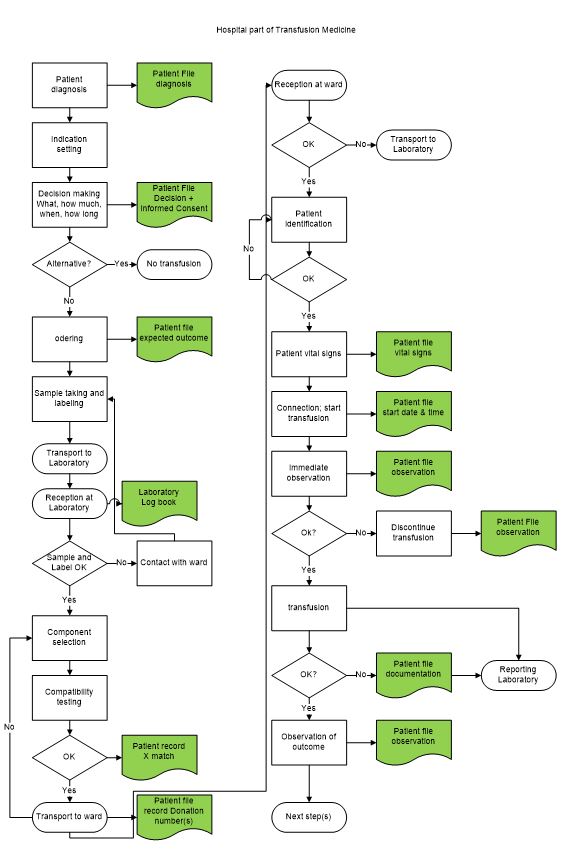
Figure 2b: In-hospital transfusion processes; Left - ordering, selection/compatibility testing; right – transfusion. Green = documentation (data collection).
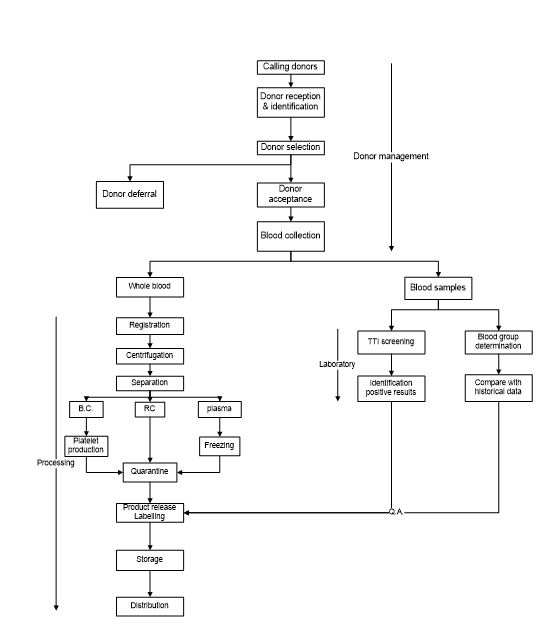
Figure 2c: Flow chart blood collection and processing. Top – donor management/blood collection; left – blood processing; right – mandatory testing.
Artificial Intelligence, Big Data and Deep Learning
In the vein-to-vein medical field of Transfusion Medicine documentation is of paramount importance. Yet, in many poor economic countries and cultures documentation is one of the least developed systems for data collection and management; numerous handwritten cahiers and files, lose paper sheets and incompletely documented request forms and records (Figure 3). In quality system management documentation is the core interconnecting all elements and steps, whether manufacture or consumption and allowing managerial and operational guidance (process descriptions, standard operating procedures) and standardization (standards), interpretation of outcomes (assessment) and initiatives to improve quality [8].
Today data and documents are mostly stored in hardware and operated through hardware networks with appropriate entrance restrictions e.g., test results, from unauthorized access.
Problems faced are the limited capacity and speed of computers, softwarde availability an maintenance, and the unreliable power supply in a considerable) part of the world.

Figure 3: Manual documentation in practice (Uzbekistan).
Noticeable advances – directions for the future?
However, over the last decade computer capacity has gone through a tremendous development which still continuous to grow. That paved the way for a machine handling of the daily mass of blood collection and processing data applying artificial intelligence by building artificial neural networks (ANN) and allowing complex layered grouping of artificial neurons process wise – input layers à hidden layers à output layer [9]. Input layers supply the ‘raw’ data; hidden layers process or compute these data mathematically using algorithms generating outcomes which appear in the output layer (Figure 4).
The more hidden layers the deeper the computational learning; the ANN becomes a DNN or deep neural network.
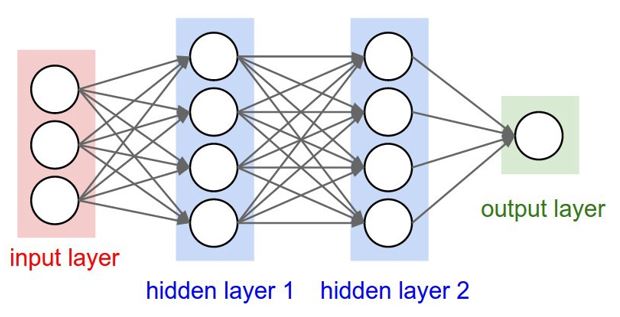
Figure 4: An example of a Deep Neural Network (DNN) with three different input artificial neurons, two hidden processing layers with each four artificial neurons and one output layer.
The magnitude of input, mathematical computations, speed of analysing and quality of outputs depends on the capacity of the hardware available, the architecture and training or conditioning of the ANN, and the infrastructural environment allowing an undisturbed computation or deep learning process.
Deep learning is a class of machine learning algorithms that uses multiple layers to progressively extract higher-level features from the raw input.
The magnitude and quality of raw data depends on the operational size or economy of scale and the existence of a well-operated quality system management of the vein-to-vein process in all its complexity of primary, supportive, and steering processes.
Deep neural networks
To build and condition such artificial DNN a meticulous and detailed analysis of all three categories of processes – primary, supportive, and steering, and their interrelations and interconnections is essential. Conditioning artificial intelligence and the needed DNN is the hardest part because you need
- a large data set (big data).
- a large amount of computational capacity and power (quantum computer).
- to condition the DNN to provide 100% correct quality outcomes.
To condition AI and its DNN one needs to give the inputs from the existing data set and compare the output or outcome with the known historic outcome of the used data set. Since at onset AI and DNN are not yet conditioned, the outcome will be wrong. Going through the whole data set you may create a function that shows how the computed outputs differ from the real outputs, a so-called Cost Function. Ideally the Cost Function should be zero. This can be achieved by adjusting the so-called weights of the neurons which could efficiently be done using a technique called Gradient Descent. This works by changing the weights in small increments after each data set iteration by computing the derivative or gradient of the Cost Function to see in which direction the minimum will be. This is a time-consuming process that needs a large computational power, where fortunately the changing of the weights is done through Gradient Descent in an automated way using quantum computers [10].
Machine learning and robotics
Machine learning refers to the ability of a machine, usually a computer, to learn using large data sets (big data) instead of using hard coded rules. This type of learning takes great advantage of the processing power and speed of the modern computer generation, able to process easily and fast large data sets (big data). The learning can be done in a supervised (using labelled data sets) or unsupervised way (using data sets with no specified structure). In the blood supply we generally use specified data sets, and therefore should apply supervised machine learning.
In the advanced world machine learning has been introduced in the clinical decision-making for transfusion, using data sets and stochastic dynamic programming [10] to predict the need [12-16]. ANNs have been shown to provide an acceptably reliable mechanism to evaluate preoperatively the potential for peri-operative transfusion across a wide range of surgical interventions, reducing unnecessary cross-matching and out of stock of blood components for a considerable amount of time during the day [15].
Pretransfusion compatibility testing is also entering AI through machine learning, with the introduction in the late 20st Century of computerized or electronic crossmatching, saving time, reagents and labour [17,18]. This is considered as safe as the immediate spin crossmatch provided there are no clinically significant alloantibodies present and there is no ABO discrepancy.
Increasingly, robotics has been introduced in almost all manufacturing processes of blood, e.g., automated mixing/weighing machines in blood collection, blood grouping and infectious disease marker testing robots using algorithms, advanced blood cell separators and centrifuges, computerized labelling machines and machine learning-based cold chain management.
Digital Foot-Printing and the Future of Transfusion Medicine
With the rapid development and extension of the use of AI with its Machine Learning and Deep Learning capacities (Figure 5), the approach might help accelerate the bridging of the global gaps existing in the blood supply and clinical use between the more advanced world and the developing world [19].
Over the past decades the most significant change has been the acceptance of Transfusion Medicine (TM) as a medical subdiscipline focusing on the patient to determine appropriate therapy. In the early years of this century greater emphasis was placed on patient blood management (PBM) [20,21], haemovigilance [22,23], cost recovery and integration in the healthcare system [24]. During this time, TM refocused on the patient through restrictive therapeutic guidelines to ensure that every blood transfusion is evidence-based and that alternative therapies are considered to reduce risk to the patient. As a global result, blood usage, especially red cell transfusion, has decreased over the last two decades, further effected by the current pandemic.
While integrated healthcare delivery evolves, efficient suppliers, including blood establishments, must become LEAN [24] and practice continuous improvement. The prime goal is efficiency embraced stewardship. A blood availability and safety digital footprint driving efficiency, safety, patient satisfaction and lower cost might be the road to the new Rome and contribute to achieving several of the 2016-2030 UN Sustainable Development Goals (SDG) (4) as well as the 2030 UN Universal Health Coverage (UHC) goal (26).

Figure 5: Artificial Intelligence (AI) structure with Machine Learning (ML) and Deep Learning (DL) elements. AI=mimicking the intelligence or behavioural patterns of humans or any other living entity; ML = a technique by which a computer can ‘learn’ from data without using a complex set of different rules. The approach is mainly based on training a model from data sets, DL = a technique to perform machine learning inspired by our brain’s own network of neurons.
How to implement and achieve?
On the hospital side to implement and achieve this, the digital footprint could reduce and prevent errors when completing a physician order using AI and RFID (radiofrequency ID) for specimen identification, reducing specimen labelling errors and errors of ‘wrong blood in tube’, appropriate transport tracking, crossmatch errors and bedside identification problems. This digital transfusion potential could create significant financial savings through an integrated vein-to-vein system, a well-functioning clinical interface.
On the blood establishment side, the digital footprint, either integrated or separate from the hospital information system (HIS), has to be transparent in data sharing, including supply chain management within the blood establishment through a well-developed supplier-customer relationship. Transparent inventory management has the potential to move the transfusion services blood supply within an integrated delivery network (IDN) to a vendor-managed inventory within the hospital. Transparency is critical to building the confidence of an IDN that will also meet the needs for unexpected emergencies. Evidently cybersecurity will be a top priority for the protection of patient/donor privacy information.
Conclusion
The future for Transfusion Medicine, within changing, and to a large extend developing, integrated healthcare systems in the developing world, depends on designing a feasible digital footprint. Such footprint should involve both the healthcare provider and the supplier, including the community that will function in a stabilized governance and socio-economic climate and environment.
Conflicts of interest
The author declares no potential conflict of interest with respect to research, authorship and/or publication of this article.
Funding
No external funding.
References
- United Nations Universal Declaration of Human Rights, 1948.
- UNDP Human Development Index, 2021.
- UNDP. Human development indices and indicators: 2018 statistical update. In: United Nations Development Programme New York, 2018.
- United Nations. Sustainable Development Goals. New York: United Nations, 2015.
- Holmberg J. The digital footprint in transfusion medicine and the potential for vein-to-vein management. Medical Laboratory Observer, 2018.
- Jansen van Galen JP, Smit Sibinga CTh. Process Management in the Vein-to-Vein Chain. In: Smit Sibinga Cees Th. editor. Quality Management in Transfusion Medicine, Nova Science Publishers. Inc, New York, NY, USA. 2013; 4: p131-85.
- Smit Sibinga CTh, Jansen van Galen JP. The Blood Supply – A special manufacturing process. In: Manufacturing Systems: Recent Progress and Future Directions. Mellal MA editor. Nova Science Publishers, NY, USA, 2020; 8: 165-186.
- van der Tuuk Adriani WPA, Smit Sibinga CTh. The Pyramid model as a structured way of quality management. Asian J Transf Sci, 2008; 2(1): 6-8.
- Raicea R. Want to know how Deep Learning works? Here’s a quick guide for everyone, 2021.
- Du SS, Lee JD, Li H, Wang L, Zhai X. Gradient Descent Finds Global Minima of Deep Neural Networks. Proceedings of the 36th International Conference on Machine Learning, Long Beach, California, PMLR, 2019; 97: 1675-1685.
- Bellman R. Dynamic programming. 1957 Princeton University Press, Princeton, USA, 2003. ISBN 978-0-486-42809-3. Dover paperback edition.
- Haijema R, van Dijk N, van der Wal J, Smit Sibinga CTh. Blood platelet production with breaks: optimization by SDP and simulation. Int J Prod Econ, 2009; 121: 464-473.
- van Dijk NM, Haijema R, van der Wal J, Smit Sibinga CTh. Blood platelet production. A novel approach for practical optimization. Transfusion, 2009; 49: 411-420.
- Levi R, Carli F, Arévalo AR, et al. Artificial intelligence-based prediction of transfusion in the intensive care unit in patients with gastrointestinal bleeding. BMJ Health Care Inform 2021; 28: e100245. doi:10.1136/bmjhci-2020-100245.
- Walczak S, Velanovich V. Prediction of perioperative transfusions using an artificial neural network. PLoS One, 2020; 15(2): e0229450. doi: 10.1371/journal.pone.0229450.
- Mitterecker A, Hofmann A, Trentino KM, et al. Machine learning-based prediction of transfusion. Transfusion, 2020; 60(9): 1977-1986.
- Guidance for Industry. “Computerized Crossmatch” (computerized analysis of the compatibility between the donor’s cell type and the recipient’s serum or plasma type). US Dept. Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, Washington DC, 2011.
- BloodBankGuy, 2021.
- Smit Sibinga CTh, Abdella YE, Seghatchian J. Poor economics - Transforming challenges in transfusion medicine and science into opportunities. Transf Aph Sci.
- Frank SM, Waters JH. Patient blood management: Multidisciplinary approaches to optimize care. AABB Press, Bethesda, MD, USA, 2016.
- Althoff FC, Neb H, Hermann E, et al. Multimodel patient blood management program based on a three-pillar strategy. A systematic review and meta-analysis. Ann Surg, 2019; 269(5): 794-804.
- Smit Sibinga CTh. Haemovigilance: An approach to risk management and control. In: Smit Sibinga C Th. and Alter HJ, editors. Risk Management in Blood Transfusion: The Virtue of Reality. Kluwer ACADEMIC Publ. Dordrecht, Boston, London. 1999: 181-189.
- De Vries RP, Faber JC, editors. Hemovigilance – an effective tool for improving transfusion safety. Wiley-Blackwell, Chichester, Sussex, UK, 2012.
- Abdella YE, Mataria A, Sajwani FH, Pourfhatollah AA, Smit Sibinga C Th. Editorial. Ensuring effective financing of blood transfusion services in support of Universal Health Coverage. EMHJ, 2019; 25(6): 371-373. doi: 10.5348/100043Z02CS2019ED
- David Mann. Creating a LEAN culture. Tools to sustain lean conversions. 2010, CRC Press, New York, NY.
- WHO Universal Health Coverage.

