Utility of BE3A Score in Predicting Outcome of Patients with Hepatitis C Related Decompensated Cirrhosis Treated with Direct Acting Antiviral Therapy
Nishat Akbar, Abbas Ali Tasneem, Raja Taha Yaseen Khan*, Sandeep Kumar, Zain Majid, Nasir Hassan Luck
Department of Hepato-gastroenterology, Sindh Institute of Urology and Transplantation Karachi, Pakistan.
Received Date: 22/11/2021; Published Date: 21/12/2021
*Corresponding author: Raja Taha Yaseen Khan, Department of Hepato-gastroenterology, Sindh Institute of Urology and Transplantation, Karachi, Pakistan.
Abstract
Introduction: Untreated decompensated hepatitis C (HCV) related chronic liver disease (CLD) leads to poor outcome due to the associated complications. The aim of this study was to ascertain the utility of BE3A score in predicting outcome of patients with HCV CLD treated with direct acting antiviral agents (DAA).
Methods: All patients with decompensated HCV CLD and detectable serum HCV ribonucleic acid (RNA) were treated with DAAs: Sofosbuvir, Daclatasvir/Velpatasvir and Ribavirin for 6 months. BE3A (Body mass index, Encephalopathy, Albumin, Ascites, Alanine aminotransferase) score was recorded before start of treatment. Baseline blood tests and Child Turcotte Pugh (CTP) were recorded before and after the use of DAA therapy.
Results: A total of 80 patients were included in the study of which 41 (51.2%) were females. Mean age was 50.2±9.1(range 32–76) years. Most of the patients 59 (73.8%) received the Sofosbuvir/Daclatasvir/Ribavirin combination. The CTP score of patients before start of DAAs was B in 68 (85%) [B7: 28, B8: 28, B9: 12] and C in 12 (15%) [C10: 7, C11: 5]. A total of 28 (35%) patients’ CTP score was reduced to A (A5: 5, A6: 32) after 6 months of treatment with DAAs. The BE3A scores before start of treatment were (0 (1), 1 (19), 2 (32), 3 (23), 4 (3), 5 (2). A BE3A score of ≥3 was significantly associated with a drop in CTP score to A5 or A6 with DAA treatment (p=0.004).
Conclusion: BE3A score is useful in predicting outcome of patients with decompensated HCV CLD. Patients with a pre-treatment BE3A score of ≥3 is likely to undergo a reduction in their CTP score from B or C to A with DAA therapy.
Keywords: BE3A score; Hepatitis C virus infection; Direct acting antivirals
Introduction
Hepatitis C Virus (HCV) infects about seventy-one million people worldwide, and the complications of chronic HCV are a major cause of morbidity and mortality [1]. Globally, genotype 1 is the most common type followed by genotypes 3, 4 and 2; while genotypes 5 and 6 account for less than 5% of the cases [2]. Chronic HCV infection eventually leads to liver fibrosis, a wound healing response to chronic liver injury, which subsequently leads to progressively increasing portal hypertension and its consequences like variceal bleeding, ascites, hepatic encephalopathy and Hepatocellular Cancer (HCC). Treatment of HCV infection can help prevent complications of cirrhosis, and hence decrease the related morbidity and mortality [3]. The degree of liver fibrosis among patients with HCV related Chronic Liver Disease (CLD) has been shown to be reduced following treatment with both oral or injectable antivirals [4-6]. A recent research shows that DAAs induced sustained virological response (SVR) is associated with a 71% reduction in HCC risk [7].
Various scoring systems including CTP and MELD have been used to predict the outcome of patients with cirrhosis [8]. Newer scoring systems were developed later, which include CTP-creatinine score, MELD-Na and United Kingdom model for end stage liver disease (UKELD) to predict outcome of patients with cirrhosis [9]. Recently, a new score (BE3A) consisting of the sum of five factors, namely: body mass index (BMI) < 25 kg/m2, absence of encephalopathy, absence of ascites, alanine transaminase (ALT) > 60 IU/l, and albumin > 3.5 g/dl, has been identified to be associated with clinical recovery from decompensated cirrhosis after DAA treatment. Patients with a BE3A score of 4 were shown to have a 75% chance of recovering to CTP class A after DAA treatment [10]. This BE3A score has also been compared with newer models incorporating other laboratory parameters including glomerular filtration rate and thrombocyte count to predict post-treatment improvement in liver function among decompensated HCV CLD patients [11].
Considerable amount of research work has been done worldwide that has supported the beneficial effects of DAAs in terms of improving the complications of hepatitis C virus infection. Also, various studies are in progress worldwide, to devise tools to predict which patients will experience the benefits of such therapy. However, little work has been done in our set up to demonstrate these advantageous effects of DAAs. The aim of our study was to determine the utility of BE3A score in predicting the outcome of patients with HCV related cirrhosis treated with DAAs.
Materials and Methods
This prospective observational study was conducted at the outpatients’ department of the Hepatogastroenterology unit of Sindh Institute of Urology and Transplantation (SIUT). The study was performed in accordance with the declaration of Helsinki declaration and approval was obtained from the institutional Ethical Review Committee (ERC). Informed consent was taken from all the patients included in the study. All patients who were diagnosed with HCV infection, based upon a reactive viral serology (HCV antibody reactive) with HCV RNA PCR detected having features suggestive of hepatic decompensation [ascites, presence of hepatic encephalopathy, history of Upper Gastrointestinal Bleeding (UGIB), spontaneous bacterial peritonitis (SBP), hepatorenal syndrome (HRS)] were included in this study. Patients who were cases of end stage renal disease, liver cancer or those who failed to comply or could not complete the total six months of therapy with DAAs were excluded.
All patients enrolled in the study were treated with direct acting antiviral (DAA) regimen currently available in the country i.e., Sofosbuvir plus Daclatasvir or Velpatasvir plus Ribavirin for a total period of 6 months. Basic demographic data, laboratory parameters (i.e., complete blood picture, serum creatinine, serum transaminases, serum albumin) along with ultrasound abdomen were done. The Child Turcotte Pugh (CTP) and the BE3A (Body mass index, Encephalopathy, Albumin, Ascites, Alanine aminotransferase) scores were calculated and recorded before and after the use of DAA therapy. Patients were followed for six months (initially, twice weekly for a month; and then monthly for the remaining five months). The frequency of follow-up was adjusted according to development of clinical symptoms or changes in the blood parameters that may require more frequent visits. The dose of Ribavirin was adjusted according to the haemoglobin (Hb) level and decreased to 200mg twice a day when it fell below 9.5gm/dl and stopped at 8.5g/dl.
The data was analysed using Statistical Package for Social Sciences (SPSS) software version 21. The mean values of the laboratory parameters before the start of treatment and at the end of treatment with DAAs were recorded. Paired t-test was used to determine any statistically significant difference between the means of the various laboratory parameters before the start and at the end of treatment with DAAs. Chi square test was employed to determine any statistically significant association between the pre-treatment BE3A score and post-treatment reduction in CTP to class A.
Results
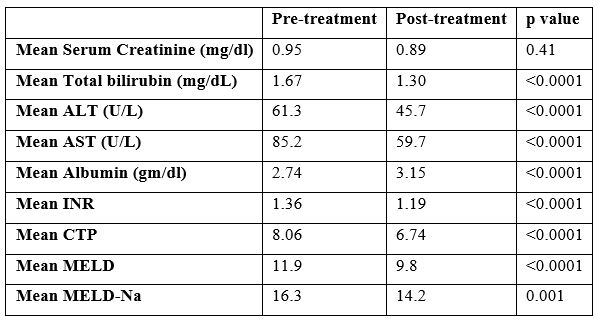
A total of 80 patients were included in the study of which half (41; 51.2%) were females. The mean age noted was 50.2±9.1(range 32 – 78) years. The mean baseline values of common laboratory parameters were as follows: Hemoglobin 10.8 gm/dl ± 2.0, serum Creatinine 0.96 mg/dL ± 0.60, Alanine aminotransferase (ALT) 61.9 U/L ± 33, Aspartate aminotransferase (AST) 86.3 U/L ± 45, white blood cells 5665/mm3 ± 2413, thrombocytes 115 ± 45, total bilirubin 1.7 ± 0.93 mg/dL and serum albumin of 2.7 ± 0.58 gm/dL. The mean post-treatment values were: hemoglobin 10.6 g/dl ± 1.9, serum creatinine 0.89 ± 0.68 mg/dl, ALT 45 ± 27.5 U/L, AST 59.5 ± 35 U/L and serum albumin 3.2 ± 0.56 g/dl. Ascites was noted in 68/80 (85%) with mild in 24/80 (30%), moderate in 31/80 (38.8%) and gross in 13/80 (16.3%). Hepatic encephalopathy was seen in 19/80 (23%). The mean pre-treatment and post-treatment CTP scores were 8.06 (±1.1) and 6.74 (±1.02) respectively. The mean pre-treatment model for end stage liver disease (MELD) score and MELD-Na were 11.9±3.97 and 16.1±5.0 while the post-treatment MELD and MELD-Na were 9.8±4.03 and 14.1±5.4 respectively. Most of the patients 59 (73.8%) received the Sofosbuvir / Daclatasvir / Ribavirin combination, followed by Sofosbuvir / Velpatasvir / Ribavirin combination 17 (21.3%); Sofosbuvir / Ribavirin 2 (2.5%) and Sofosbuvir / Daclatasvir alone 2 (2.5%). The pre-treatment BE3A scores were 0 (1), 1 (17), 2 (32), 3 (22), 4 (3), 5 (2). (Figure 1).

Figure 1: Frequency of patients according to BE3A score (n=80).
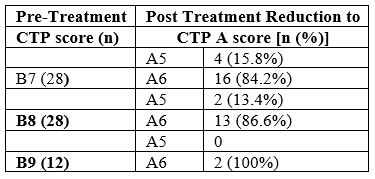
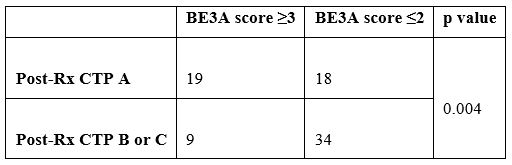
The pre-treatment CTP scores were: B in 68 (85.0%) [B7:28, B8:28, B9:12] and C in 12 (15%) [C10:7, C11:5]. Post-treatment CTP score of 37 (46.3%) patients reduced from B or C to A (A5:5, A6:32). The percentage of CTP class among patients with HCV related decompensated cirrhosis before and after completion of treatment is shown in Figure 2. The pre-treatment CTP score distribution of patients whose post-treatment CTP dropped down to class A is shown in Table 1. As shown, all those whose CTP score dropped down to class A belonged previously to CTP class B (pre-treatment); that is, none of the CTP class C patients achieved a fall in their CTP score to class A group. A comparison made between the pre- and post-treatment mean values of the laboratory parameters showed that there was a significant drop in the values of total bilirubin, AST, ALT, Albumin, PT INR, CTP, MELD and MELD-Na after treatment with DAAs (Table 2). A pre-treatment BE3A score of ≥3 was associated with a drop in CTP score to A5 or A6 with DAA treatment (p=0.004) (Table 3). With a BE3A score ≥3, the reduction in CTP to class A can be predicted with a sensitivity of 51.3%, specificity 79%, positive predictive value 68% and negative predictive value 65%.
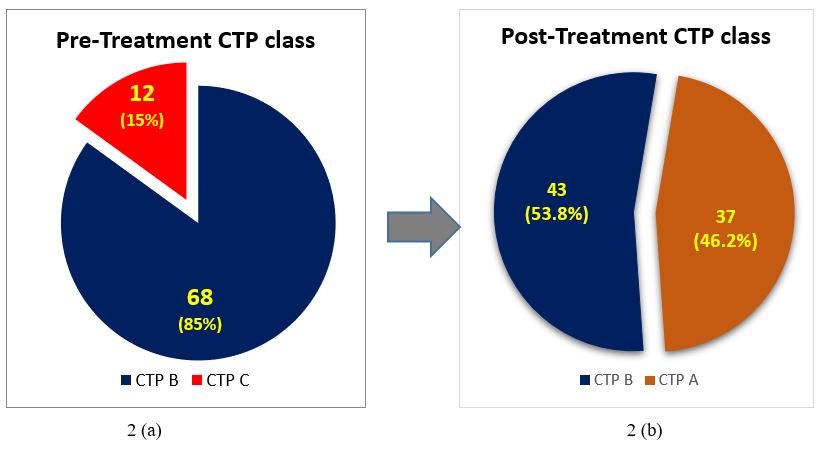
Figure 2: Reduction in the CTP class after treatment with Direct acting antivirals from CTP B and C to CTP A and B.
Table 1: Pre-treatment CTP scores of patients whose post-treatment CTP score dropped to A (n=36).
Discussion
Cirrhotic patients should be given high priority for treatment because viral clearance not only halts the progression of the disease by regression of fibrosis, but also reduces the need for liver transplantation and improves survival [12]. Regression of fibrosis in patients with decompensated cirrhosis secondary to hepatitis C virus infection has been demonstrated in various studies worldwide [4-6]. An Egyptian study by Hanan Soliman showed that the BMI, degrees of hepatic stiffness and steatosis were related to regression of hepatic fibrosis after therapy [13]. Besides, an American study by James McPhail, showed that fibrosis regression is more likely to occur in patients with advanced stages of fibrosis and less likely in patients who are obese [14]. Furthermore, an Italian study by Vincenza Calvaruso, on patients with compensated and decompensated HCV related cirrhosis, showed that achievement of SVR to DAA treatment decreased the incidence of HCC over a mean follow-up of 14 months [15].
Studies have also been done to determine the role of various scoring systems in predicting outcome of HCV CLD patients. An Indian study by Yogesh Chawla showed that when comparing CTP with MELD score, MELD more accurately predicts mortality in cirrhosis and is better than CTP for predicting the short-term and intermediate-term mortality. It also showed that adding serum creatinine to CTP, significantly improves its diagnostic accuracy for short-term mortality; however, it still remains lower than MELD alone [16]. In contrast, another study by Maria Kalafateli from Greece showed that CTP score and CTP-creatinine score have better prognostic value compared to MELD score, MELD-Na score, and United Kingdom model for end stage liver disease (UKELD) score for predicting short- and long-term mortality in patients with stable cirrhosis [9].
Recently, Omar El-Sherif et al. devised the BE3A score consisting of three bedside clinical features (BMI, absence of encephalopathy, absence of ascites) and two common laboratory tests (ALT, albumin) and showed that a BE3A score of 4 was associated with 75% chance of recovering to CTP class A after DAA treatment [10]. In our study, we utilized the BE3A score to ascertain its utility in predicting recovery to CTP class A from decompensated cirrhosis among HCV CLD patients treated with DAAs. Our analysis revealed that a score of 3 or more could also predict the recovery of decompensated cirrhosis to CTP A (p=0.01). The BE3A score has also been used in a study done in Punjab Pakistan, which showed its ability to predict outcome in those patients having decompensated liver disease secondary to Hepatitis C who are treated with DAAs therapy [17]. It also showed that this score predicted the change in the CTP and MELD but not the CTP class. Majority of the patients with scores of 3 and 4 had improved outcomes as evidenced by a reduction in their MELD scores but no patient had a change of their CTP class to A. However, in our study, 46.7% patients experienced a drop-in CTP class from either B or C to A. It is interesting to know the reason for this discrepancy between their and our results. One reason could be the difference in the genotype of the HCV between the two cities of the same country. The other reason could be the difference in the quality of DAAs used among the two patient populations. However, further studies with larger sample sizes may be required to fully determine the reason for the difference in the results.
Conclusion
BE3A score is useful in predicting outcome of patients with decompensated HCV CLD. Patients with a pre-treatment BE3A score of ≥3 is likely to undergo a reduction in their CTP score from B or C to A with DAA therapy.
Conflict of Interest
The authors hereby confirm that there is no conflict of interest
References
- Holmes JA, Rutledge SM, Chung RT. Direct-acting antiviral treatment for hepatitis C. Lancet. 2019; 393(10179): 1392-1394. doi: 10.1016/S0140-6736(18)32326-2. Epub 2019 Feb 11. PMID: 30765125.
- Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol, 2016; 22(34): 7824-7840. DOI: 10.3748/wjg.v22.i34.7824. PMID: 27678366; PMCID: PMC5016383.
- Basit H, Tyagi I, Koirala J. Hepatitis C. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2020.
- Rockey DC. Fibrosis reversal after hepatitis C virus elimination. Curr Opin Gastroenterol, 2019; 35(3): 137-144. DOI: 10.1097/MOG.0000000000000524. PMID: 30865043; PMCID: PMC6535048.
- Cheng CH, Chu CY, Chen HL, Lin IT, Wu CH, Lee YK, et al. Direct-acting antiviral therapy of chronic hepatitis C improves liver fibrosis, assessed by histological examination and laboratory markers. J Formos Med Assoc. 2020: S0929-6646(20): 30595-30597. DOI: 10.1016/j.jfma.2020.11.018. Epub ahead of print. PMID: 33339709.
- El-Raziky M, Khairy M, Fouad A, Salama A, Elsharkawy A, Tantawy O. Effect of Direct-Acting Agents on Fibrosis Regression in Chronic Hepatitis C Virus Patients' Treatment Compared with Interferon-Containing Regimens. J Interferon Cytokine Res. 2018; 38(3): 129-136. DOI: 10.1089/jir.2017.0137. Epub 2018 Mar 12. PMID: 29565743.
- Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017: S0168-8278(17): 32273-32280. DOI: 10.1016/j.jhep.2017.08.030. Epub ahead of print. PMID: 28887168; PMCID: PMC5837901.
- Fernández Carrillo C, Lens S, Llop E, Pascasio JM, Crespo J, Arenas J, et al. Treatment of hepatitis C virus infection in patients with cirrhosis and predictive value of model for end-stage liver disease: Analysis of data from the Hepa-C registry. Hepatology. 2017; 65(6): 1810-1822. DOI: 10.1002/hep.29097. Epub 2017 Apr 28. PMID: 28170112.
- Kalafateli M, Zisimopoulos K, Vourli G, Rigamonti C, Goulis J, Manesis E, et al. Prognostic Models for Survival in Patients with Stable Cirrhosis: A Multicenter Cohort Study. Dig Dis Sci. 2017; 62(5): 1363-1372. DOI: 10.1007/s10620-017-4504-3. Epub 2017 Mar 1. PMID: 28251503
- El-Sherif O, Jiang ZG, Tapper EB, Huang KC, Zhong A, Osinusi A, et al. Baseline Factors Associated With Improvements in Decompensated Cirrhosis After Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection. Gastroenterology. 2018; 154(8): 2111-2121.e8. doi: 10.1053/j.gastro.2018.03.022. Epub 2018 Mar 11. PMID: 29535028.
- Debnath P, Chandnani S, Rathi P, Nair S, Junare P, Udgirkar S, et alA, Contractor Q. A new model to predict response to direct-acting antiviral therapy in decompensated cirrhotics due to hepatitis C virus. Clin Exp Hepatol. 2020; 6(3): 253-262. doi: 10.5114/ceh.2020.99525. Epub 2020 Sep 30. PMID: 33145432; PMCID: PMC7592091.
- Velosa J. Why is viral eradication so important in patients with HCV-related cirrhosis? Antivir Ther. 2017; 22(1): 1-12. DOI: 10.3851/IMP3077. Epub 2016 Aug 24. PMID: 27553973.
- Soliman H, Ziada D, Salama M, Hamisa M, Badawi R, Hawash N, et al. Predictors for Fibrosis Regression in Chronic HCV Patients after the Treatment with DAAS: Results of a Real-world Cohort Study. Endocr Metab Immune Disord Drug Targets. 2020; 20(1): 104-111. doi: 10.2174/1871530319666190826150344. PMID: 31448717.
- McPhail J, Sims OT, Guo Y, Wooten D, Herndon JS, Massoud OI. Fibrosis improvement in patients with HCV treated with direct-acting antivirals. Eur J Gastroenterol Hepatol. 2020. DOI: 10.1097/MEG.0000000000001821. Epub ahead of print, PMID: 32639414.
- Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, et al. Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated with Direct-Acting Antiviral Agents. Gastroenterology. 2018; 155(2): 411-421.e4. DOI: 10.1053/j.gastro.2018.04.008. Epub 2018 Apr 12. PMID: 29655836.
- Chawla YK, Kashinath RC, Duseja A, Dhiman RK. Predicting Mortality Across a Broad Spectrum of Liver Disease-An Assessment of Model for End-Stage Liver Disease (MELD), Child-Turcotte-Pugh (CTP), and Creatinine-Modified CTP Scores. J Clin Exp Hepatol. 2011; 1(3): 161-168. doi: 10.1016/S0973-6883(11)60233-8. Epub 2012 Jan 2. PMID: 25755381; PMCID: PMC3940129.
- Nawaz Arif, Riaz Saherish, Iqbal Haseeb, Khan Zohaib, Ali Bushra, Chowdhry Asad, et al. Can BE3A Score Predict Improved Outcomes in Patients With Decompensated Cirrhosis Receiving Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection? The American Journal of Gastroenterology: 2019; 114: p S1614. DOI: 10.14309/01.ajg.0000601424.27307.1e.

