Primary Prevention of Cardiovascular Events in the Elderly; Does LdlLowering Therapy Outweigh the Potential Side Effects?
Frederique Burger, Jan Westerink, Michiel L Bots, Monika Hollander
Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University,
Netherlands.
Department of Vascular Medicine, University Center Utrecht, Netherlands.
Julius Center for Health Sciences and Primary Care, Department of General Practice, University Center Utrecht, Netherlands.
Received Date: 19/11/2021; Published Date: 17/12/2021
*Corresponding author: Michiel L Bots, MD, PhD, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands.
Abstract
Background: Recommendations for LDL-lowering treatment in the elderly ≥75 years in primary prevention are less direct than in secondary prevention patients. Therefore, initiation of this treatment heavily depends on the physician-patient discussion and remains arbitrary. This study aimed to conduct an accurate assessment of the newest available evidence of the efficacy of LDL-lowering therapy in elderly ≥75 years without established CVD (i.e., primary prevention) versus the most recent available evidence of side-effects to make the physician- patient discussion easier and purely factual.
Methods: We searched the PubMed database up to December 3th on efficacy of LDL- lowering treatment in the elderly in primary prevention and performed another search concerning side-effects of LDL-lowering therapy/statin treatment. We presented results of large recent meta-analyses, individual trials and cohorts. The quality of methodology was generally assessed by the Cochrane Risk of Bias tool and we took these as quality indicators.
Results: Direct evidence from RCTs and meta-analyses of significant CV-risk reduction by LDL-lowering therapy in the specific group of the elderly ≥ 75 years in primary prevention remains absent. However, evidence from cohort studies show significant reduction in CV-risk. No trial evidence shows a causal relationship between statin treatment and adverse events, except for new-onset Diabetes (OR 1.09, 95%CI 1.02-1.17).
Conclusion: Evidence points in the direction of benefit of LDL-lowering treatment in the elderly in primary prevention. If life-expectancy is long enough, there are no signs of frailty and the patient feels comfortable after the shared decision, with no evidence of a causal relationship with side-effects, the absolute benefit seems to outweighs the risk.
Introduction
The issue
A 78-year-old male patient came to the General Practitioner (GP) for a blood pressure follow- up. His medical history includes hypertension, hypothyroidism, slightly impaired stable renal function (G3aA2) and prostate carcinoma with radical prostatectomy. Current medication consists of Levothyroxine, Irbesartan, and Nifedipine. He is a lifelong non-smoker, occasional alcohol drinker and manages to live a healthy lifestyle without obvious signs of frailty. Family history contains no premature cardiovascular diseases. Physical examination shows a BP (blood pressure) of 145/85 mmHg and a BMI (Body Mass Index) of 27 kg/m2. Blood results were as followed; fasting glucose level 5.1 mmol/L, Total Cholesterol 7,0 mmol/L, HDL-cholesterol 1.7 mmol/L, LDL-cholesterol 5 mmol/L, MDRD- eGFR 59 ml/min/1,73m2 and a urine Albumin/Creatinine ratio (ACR) of 8.0 mg/mmol.
Because of this patient’s slightly impaired stable renal function with moderately increased albuminuria (G3aA2), especially in combination with his age, this patient is at high risk of developing a cardiovascular event as stated by table 1; Dutch Cardiovascular Risk Management guideline [22].
According to the current Dutch guidelines and international KDIGO guidelines, life style intervention is indicated and drug intervention could be considered to decrease the CV -risk in a high-risk non-frail patient above the age of 70 years.1 Importantly, the ESC-guideline apprises only to start therapy when the benefit appears to outweighs the risk of adverse events (apart from drug-drug interaction and costs) after a patient-discussion and do not mention frailty. One way to reduce the patient risk with medication, is starting LDL-lowering therapy [2]. This recommendation is less direct than recommendations for secondary prevention patients in this age group and therefore heavily depends on the physician-patient discussion. In order to be able to make a well-considered decision, evidence of benefit of treatment and potential harms should be clear.
Although the efficacy of LDL-cholesterol lowering with statin is proven to reduce the CV-risk by around 20% per 1 mmol/L reduction LDL in both primary and secondary prevention, the absolute benefit of this treatment in elderly above 75 years of age without known cardiovascular diseases remains equivocal [3,4]. This is in part due to the lack of representation of this particular population in clinical trials and because of the limited data on the absolute benefit in term of (CVD-free) life years gained.
Apart from the limited data about benefit of statin in elderly patients without established CVD, this is also the case for side-effects associated with statin therapy. Although there is only very limited evidence from randomized controlled trials for real statin related side effects, presumed adverse events are widely discussed in media and therefore by patients and physicians. Because perceived side-effects could cause a significant negative influence on quality of life and influence self-sufficiency, this might have a substantial effect on the benefit/risk ratio of this therapy. Especially in the elderly population, it plays a crucial role during the shared decision-making process. Therefore, evidence of side-effects is an important factor to consider during the shared decision-making process and will be discussed.
The aim of this study is to make the physician-patient discussion about LDL-lowering therapy easier. Therefore, this paper aimed to conduct an accurate assessment of the newest available evidence of the efficacy of LDL-lowering therapy in elderly ≥75 years without established CVD (i.e., primary prevention) versus the most recent available evidence of side-effects. To accomplish the aim of this study, we defined the following research question: ‘To which extent does CV-risk reduction with lipid lowering therapy outweigh the potential side-effects in not fragile elderly ≥ 75 years during primary prevention?’.
Methods
Search for evidence on efficacy
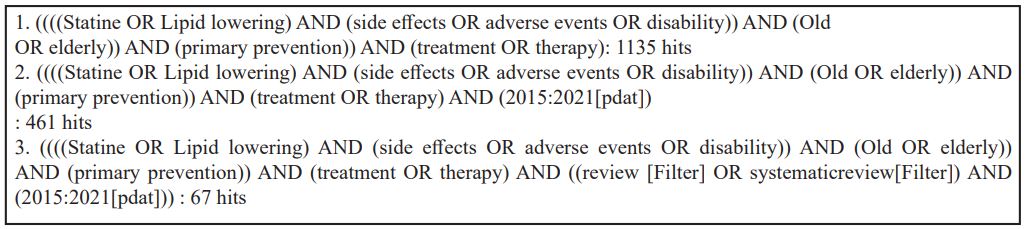
We started to evaluate current guidelines; NHG, FMS, ESC/EAS, NICE and ACC/AHA (See appendix 1). Whereupon, we searched in the PubMed database for systematic reviews regarding efficacy of lipid- lowering, published up to December 1, 2020. We used the following search terms: Lipid lowering, efficacy, elderly and primary prevention. (See appendix 2a for complete search strategy)
Search for evidence on side-effects
Next, we conducted a second search in the PubMed database for (systematic) reviews concerning potential side effects of lipid-lowering therapy, published up to December 3, 2020. We used the following search terms: lipid-lowering therapy, disability, side-effects, elderly and primary prevention. (See appendix 2b)
RCT’s and Cohorts
Additionally, we used the original references mentioned in the systematic reviews and scanned the ‘related articles’ showed by PubMed on title and abstract.
Systematic reviews and articles were selected by predefined inclusion and exclusion criteria. (appendix 3). We considered systematic reviews with RCT’s for the efficacy of LDL-lowering therapy. Due to the lack of representation of elderly ≥75 years without established CVD in RCT’s, meta-analyses and results from systematic reviews were not always applicable to our study population. Therefore, we also included individual studies other than RCT designs, such as cohorts.
The primary outcomes were CV-risk reduction (%) and side-effect risk (%)
The quality of evidence in meta-analysis was generally assessed in the reviews. Methodology used was the Cochrane Risk of Bias tool. We considered the reported findings as quality indicators.
Results
Efficacy LDL-lowering in elderly
Cohort evidence
Due to limited data from RCT’s and meta-analyses of efficacy of CV-risk reduction by statin treatment in elderly ≥ 75 years in primary prevention, we looked for evidence based on non-randomised studies. A cohort study of US veterans ≥ 75 years (n=326981) without established cardiovascular disease receiving new statin therapy had been performed. The aim of this study was to evaluate the CVD- mortality and all-cause mortality. After propensity score weighting was applied, statin use was associated with a lower risk of all-cause mortality and cardiovascular mortality, (HR 0.75, 95%CI: 0.74- 0.76) respectively (HR 0.80, 95%CI: 0.78-0.81). In sub analyses of different age groups, a significant risk reduction was still observed, even in those ≥90 for all cause-mortality and cardiovascular death (HR 0.80, 95%CI: 0.74-0.87) and (HR 0.81, 95%CI: 0.70-0.94), respectively. Stratification by race or diabetes yielded no significant differences in primary outcomes. Results for secondary outcomes were as follows; composite of atherosclerotic cardiovascular disease (HR 0.92, 95%CI: 0.91-0.94), Myocardial infarction (HR 0.99, 95%CI: 0.97-1.03), ischaemic stroke (HR 0.98, 95%CI:0.96-1.01) and revascularisation (HR 0.89, 95%CI: 0.88-0.91) [15].
Trial evidence
A systematic review performed in 2015 by Teng M and co-workers, compared any statin treatment with placebo or usual care for primary prevention in adults >65 years. Eight randomized controlled trials were included (n= 25952) of which six were double blinded and two open blinded. Patients had no history of cardiovascular disease, but differed in prevalence of diabetes. Overall proportion of diabetic patients in this meta-analysis was 51.2%. Primary outcome was defined as an extended composite major adverse cardiovascular event [MACE]; myocardial infarction, stroke, coronary revascularization, cardiac sudden death and angina. Quality of methodology was assessed for all included trials using the Cochrane Risk of Bias tool. The overall outcome was reported as moderate quality [1].
Results showed a significant decrease of incidence of MACE (RR 0.82, 95%CI: 0.74-0.92) when using a statin. This was also seen for non-fatal myocardial infarction (RR 0.75, 95%CI: 0.59-0.94) and total myocardial infarction (RR 0.74, 95%CI: 0.61-0.90). Statins did not statistically significantly reduce the risk of fatal MI (RR 0.43, 95%CI: 0.09-2.01), total stroke (RR 0.85, 95% CI: 0.69–1.06) and all-cause mortality (RR 0.96, 95%CI: 0.88-1.04) [1].
The recently published meta-analyses by Gencer and co-workers partly supported the results of Teng. They included 6 articles to evaluate available evidence from randomized controlled trials, for patients
≥75 years old (n=21492) from March 1, 2015 up to Aug 14, 2020. Most data were extracted from the CTT Collaboration meta-analyses (n=24) and a handful were individual trials (n=5). From the 29 included trials, 25 concerned statin trials and four concerned non-statin trials. Quality of methodology had been assessed for all included trials by the Cochrane Risk of Bias tool. Most trials were categorized as low rate of bias, except for EWTOPIA-75 which scored moderate bias in two components; performance- and attribution bias [2].
Results showed that decreasing LDL cholesterol significantly reduces the risk of major vascular events in older patients by 26% per 1 mmol/L reduction in LDL cholesterol (RR 0.74, 95%CI: 0.61–0.89). No significant differences in risk reduction between patients younger than 75 years (RR 0.85, 95%CI: 0,78– 0,92] and older patients were observed [2]. However, these results refer to older patients with- and without established CVD. In other words, primary and secondary prevention combined.
The individual patient data received from the CTT-database and EWTOPIA trial allowed a sub analyses for older patients without established CVD. The EWTOPIA trial did find a significant risk reduction in elderly >75 years for primary prevention. They observed Asian patients who received ezetimibe and dietary counselling versus the control group who received usual care. They reported a RR of 0.36, (95%CI; 0.18- 0.69) per 1 mmol/L reduction in LDL-c. This trial had an open-label design, early termination and issues with follow-up. Although this is a non-statin trial (ezetimibe), it focused on CV- risk reduction with LDL-lowering therapy in patients >75 in primary prevention setting and may be a useful outcome to evaluate [2,4].
However, the CTTC sub analysis for primary prevention patients showed no significant risk reduction of CVD per 1 mmol/L reduction of LDL. Patients ≥75 years who received LDL-lowering therapy or intensive statin versus the control group with placebo or less intensive statin showed a RR of 0.92 (95%CI; 0.77-1.10). However, this trial contained too few patients in primary prevention (i.e., free from previous CVD) in this specific age-group to do a reliable assessment with sufficient precision [2,3].

Side Effect
Cohort evidence
A nested-case control study (n= 252460) assessed the risk factors for development of rhabdomyolysis in new users of lipid-lowering therapy. Whether these patients were known with cardiovascular or had no history of cardiovascular disease at baseline was not reported. Because this adverse event has a very low incidence in society, focus lies on statin treatment and occurrence of rhabdomyolysis instead of the setting of primary or secondary prevention. Twenty-one cases of rhabdomyolysis were identified by reviewing medical records and compared with two hundred reference individuals. Age appeared to be the most important risk factor. Patients ≥ 65 years had a four times higher risk of developing rhabdomyolysis than patients under 65 years (OR 4.36, 95%CI: 1.45-14.13) [5,6].
In patient registries, muscle related symptoms account for 7-29% in statin-users [8,11]. An internet survey of 10.138 US of former and current statin users was conducted. Patients were ≥18 years old (mean age 61 years) with registered high cholesterol levels by their health care provider or self-reported diagnosis. Results showed that muscle symptoms were the most common reason for statin discontinuation (60%), statin non-adherence (52%), and statin switching (33%). Demographic characteristics were shown, but no sub analysis for primary prevention patients was available [8,18].
Trial evidence
Teng and co-workers presented results on the risk of side-effects based on the trials reported in their systematic review. No age-effect analysis was presented. Significant differences between statin treatment and placebo were not observed. They evaluated myalgia (RR 0.88, 95%CI: 0.69-1.13), elevation of hepatic transaminases (RR 0.98, 95%CI: 0.71-1.34), new-onset diabetes (RR 1.07, 95%CI: 0.77-1.48), serious adverse events (RR 1.00, 95%CI: 0.97-1.04) and discontinuation due to (not further specified) adverse events (RR 1.10, 95%CI: 0.85-1.42). Risk of myopathy, rhabdomyolysis and cognitive impairment were not described in this study due to the lack of reports in the included trials [1].
Another meta-analysis from Zhen et al. (n= 18192, mean age 73.3) aimed to evaluate safety in elderly
≥ 65 year without established CVD from data of 11 double blinded randomized controlled trials. This meta-analysis focuses on muscle-related symptoms but also reported total adverse events and serious adverse events. Whereas muscle related symptoms were defined as myalgia, weakness, tenderness, stiffness and cramp; adverse events were not further specified. Serious adverse events were life threatening events, permanent disability or hospitalisation. Compared with placebo, statins did not increase the risks of muscle-related symptoms (RR 1.01; 95% CI 0.90-1.12), total adverse events and serious adverse events. There was no association with more permanent treatment discontinuation due to side-effects [7].
In the ‘Older people’ section of the scientific statement from the American Heart Association, no differences in side effects between older people in the control/placebo group compared with elderly who received treatment was described [5]. This scientific statement was partially based on the results of the randomized controlled PROSPER-trial (n=5804) which compared pravastatin 40 mg to placebo in men and women 70 to 82 years of age with or at high risk (due to smoking, hypertension or Diabetes) for vascular disease. Serious adverse event rates were similar between groups; 55% (n= 1604) reported adverse events in placebo group versus 56% (n=1608) in the statin group. Myalgia was observed in 32 patients in placebo group versus 36 patients in statin group. No cases of rhabdomyolysis, myopathy (10 times the upper limit of creatine kinase) and increased hepatic transaminases were reported [19]. They also mentioned a secondary analysis of the JUPITER STUDY, a double blinded randomized controlled trial, which analysed results for adverse events in patients without established CVD in patients >70 years (n=5695). No statistic significant differences between statin and placebo were observed; muscle weakness, stiffness or pain (HR 1.04, 95%CI: 0.92-1.19), Myopathy (HR 1.31, 95%CI: 0.29-5.84), hepatic disorder (HR 1.01, 95%CI: 0.71-1.45) and any serious adverse events (HR 1.05, 95%CI: 0.93-1.17) [15]. Lastly, the SEARCH trial with patients (n= 12000) between 18-80 years old with myocardial infarction in medical history and currently on statin therapy was described. This trial did an assessment of risk factors for development of myopathy. To gain enough statistical power, they combined incipient myopathy and myopathy cases. Because 98 cases were identified and this number is substantially larger than other trials (i.e., PROSPER and JUPITER), a risk factor assessment was possible and therefore may be relevant. Results showed that age >65 years was associated with an approximately doubled rate of statin-induced myopathy/incipient myopathy. They assumed that a similar result would have been derived, when using enough single myopathy cases. Yet this outcome concerns elderly with known cardiovascular disease and no sub analysis of patients >75 years is available [7].
Although muscle-symptoms are critically important side-events, new- onset diabetes is also a concern associated with statins. Especially during prescription in elderly where no disease has presented yet. A meta-analysis showed that statin therapy was associated with a 9% (OR 1.09, 95%CI 1.02-1.17) increased risk for new-onset diabetes.8,9 Additionally, the diabetes risk is associated with an increase of 12% (OR 1.12, 95%CI 1.04-1.22) comparing the use of high and low dose statin therapy [8,10].
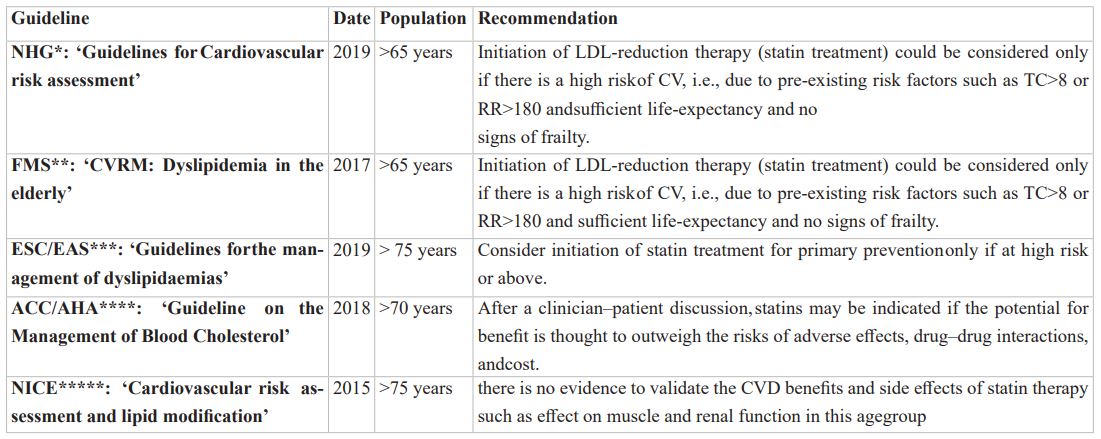
* Dutch General Practioner Association
**Federation of Medical Specialists in the Netherlands
*** Risk reduction in cardiovascular events from those who have received statin
*** European society of cardiology/ European Atherosclerosis Society
**** National Institue for Health and Care Excellence
***** American College of Cardiology/ American Heart Association
Appendix 2: Search strategies
Table 2a: Efficacy lipid-lowering in primary prevention.

Discussion
Efficacy of LDL-lowering therapy in elderly, both in primary and secondary prevention combined has shown a significant lower CV-risk. Direct evidence of efficacy of reducing cardiovascular risk in primary prevention in patients ≥75 years alone, still remains rare in results of RCTs [1-3]. Although RCTs are considered as most reliable study designs to prove a causal relationship of efficacy, data are too limited to do a reliable assessment in this specific population of elderly patients above the age of 75 years without cardiovascular disease at baseline. The ongoing STAREE trial will determine whether results of efficacy will be as strong in not fragile elderly above 70 years without cardiovascular disease as in elderly in secondary prevention. In addition, it will evaluate CVD-free survival and disability free survival and therefore give more insight in the net benefit of statin treatment [20].
Other study designs however, e.g. (retrospective) cohorts, showed more clear results in this particular age group without established CVD. Despite the potential risk of bias due to unknown confounders and confounding by indication in these study designs, it allows an evaluation of large amount of routine clinical practice patients. Additionally, circumstantial evidence strengthens results of previously described cohorts. In a primary prevention cohort of participants ≥75 years, discontinuation of statin was associated with a 33% increased risk for cardiovascular event hospital admission [14]. Therefore, evidence of efficacy of LDL-lowering therapy in elderly for primary prevention points into the direction of benefit. Conclusively, LDL-lowering therapy may need a more prominent role in primary prevention in elderly than currently advised in guidelines and weigh heavier in physician-patient discussion.
However, beneficial risk ratios of CV-risk reduction are not similar to absolute benefit of treatment.
Apart from efficacy, patient may decide to refrain from treatment based on alleged side-effects, due to the widespread negative news of statins. However, results of RCTs never showed any causal relationship. Age is found to be a risk factor to develop myopathy and rhabdomyolysis but the main cause may not be statin-treatment itself, but a higher risk due to drug-drug interaction and different pharmacokinetics and- dynamics in the elderly which leads to these otherwise very rare side effects.
However, LDL-lowering treatment (statin) is associated with a higher risk of new-onset Diabetes. In absolute numbers, this means one additional case of diabetes in every 498 patients versus one prevented cardiovascular event for 155 patients treated (each year). Furthermore, patients who developed DM type 2 while receiving treatment had a lower rate of macro- and micro vascular complication. The benefit of preventing a CV-event therefore seems to outweigh the risk at new-onset (mild) Diabetes [8,10].
From patients’ registries and surveys, muscle-related symptoms are responsible for statin discontinuation, switching or non-adherence. Although this problem reflects a problem in daily practice, a plausible explanation is that physicians or patients wrongfully link muscle-discomfort (which is more prevalent in elderly) to statin-treatment. Therefore, quit or switch treatment without a justified reason while at the same time increasing the nocebo effect during rechallenge. Fear of side-effects therefore may be overrated [13].
A helpful tool to quantify the CV-risk in an individual is presented on U-prevent.com [21]. When using the elderly risk score, this tool sketches the 10-year CV-risk without treatment. The elderly risk score was specifically developed for elderly patients, as using the general SCORE-model (whole population) tended to overestimate the 10-year risk in elderly patients. This tool can estimate the influence of LDL-lowering therapy in CV-risk reduction in terms of absolute risk reduction and NNT over a certain timespan. Because statins in primary prevention are estimated to take two to five years to be effective, life expectancy should be enough to be able to benefit from treatment [12]. Therefore, the CV life-time risk and CV life-time risk model may be more useful. The LIFE-CVD model (also available on u- prevent.com) is the most suitable in this case (among all others options based on patient characteristics). Using the LIFE-CVD model provides even more accurate predictions of long-term risk as it also takes competing mortality into account. These life time predictions and hazard ratios from the trials together, can be used to estimate the absolute benefit in CVD-free life years gained after treatment initiation.
If we use this LIFE-CVD model for our patient, the 10-year risk of development of CVD is 20%. It shows a life-time risk of 22% without treatment. With statin therapy (atorvastatin 20mg 1dd), a 6.7% reduction in CV-risk can be expected with 15 number needed to treat. The lifetime benefit is estimated to be approximately +1 year of CVD-free survival.
Because this tool is helpful to quantify life time benefit of treatment and estimates CV-risk reduction for10 years, it can be used during physician-patient discussion. However, it is a estimation and should not be leading subject of the discussion.
Strengths and Limitations
Because this review mostly evaluated outcomes of systematic reviews with large meta-analysis, it was hard to maintain a structured overview of patient characteristics, e.g., diabetic or non-diabetic, frail or vital. To obtain more patient specific results, a new IPD meta-analyses might have been better.
Although data of CTT (which is an IPD) was used, too little data of primary prevention was available to do a reliable assessment. Additionally, an IPD of side-effects could also be useful.
This review mainly focuses on statins because its current position as cornerstone in treatment of LDL- lowering. Nowadays there are other drugs available that can reduce LDL sufficiently with a different pathway, e.g., PCSK-9 inhibition. These drugs have not been assessed in this study, but might offer a greater benefit/risk ratio in the future. The same applies for Ezetimibe treatment. Although the EWTOPIA study had its limitations, it showed that Ezetimibe succeeded in reducing CV-risk and is not associated with adverse events. Current guidelines advise to consider adding Ezetimibe to current statin treatment, only when target LDL-levels are not achieved by statin treatment [16]. Further trials might help to evaluate effectiveness of single-ezetimibe treatment and might offer a greater absolute benefit for the elderly in the future.
In conclusion, direct evidence from RCTs remain scarce, but other study designs give indication that LDL-lowering therapy is effective and beneficial in the elderly in primary prevention. The STAREE trial will hopefully present more direct evidence of efficacy and disability free survival in this specific population and should give more certainty. If life-expectancy is long enough and the patients feels comfortable after the shared decision, together with the fact there is no evidence of a causal relationship with side-effects, the absolute benefit seems to outweighs the risks.
References
- Teng M, Liang Lin, Ying Jiao Zhao, Ai Leng Khoo, Barry R Davis, Quek Wei Yong, et al. Statins for Primary Prevention of Cardiovascular Disease in Elderly Patients: Systematic Review and Meta-Analysis. Drugs aging, 2015; 32(8): 649-661. Doi: 10.1007/s40266-015-0290-9.
- Gencer B, Marston NA, Im K, Cannon CP, Sever P, Keech A. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2020; 396(10263): 1637-1643. Doi: 10.1016/S0140- 6736(20)32332-1.
- Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019; 393(10170): 407-415. DOI: 10.1016/S0140-6736(18)31942-1.
- Ouchi Y, Sasaki J, Arai H, Yokote K, Harada K, Katayama Y, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. 2019; 140(12): 992-1003. Doi: 10.1161/CIRCULATIONAHA.118.039415.
- Newman CB, Preiss D, Tobert JA, Jacobson TA, Page RL, Goldstein LB, et al. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Bio, 2019; 39(2): e38-e81. DOI: 10.1161/ATV.0000000000000073.
- Schech S, Graham D, Staffa J, Andrade SE, La Grenade L, Burgess M, et al. Risk factors for statin-associated rhabdomyolysis. Pharmacoepidemiol Drug Saf. 2007; 16: 352-358. Doi: 10.1002/pds.1287.
- Zhou Z, Albarqouni L, Curtis AJ, Breslin M, Nelson M, et al. The Safety and Tolerability of Statin Therapy in Primary Prevention in Older Adults: A Systematic Review and Meta-analysis. 2020; 37(3): 175-185. Doi: 10.1007/s40266-019-00736-y
- Ferri N, Corsini A. Clinical pharmacology of statins : an update. Curr Atheroscler Rep. 2020; 22(7): 26. Doi: 10.1007/s11883-020-00844-w.
- Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010; 375(9716): 735-742.
- Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011; 305(24): 2556–2564.
- Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015; 36(17): 1012-1022. Doi: 10.1093/eurheartj/ehv043.
- Orkaby AR, Driver JA, Ho YL, Lu B, Costa L, Honerlaw J, et al. Association of Statin Use with All- Cause and Cardiovascular Mortality in US Veterans 75 Years and Older. JAMA. 2020; 324(1): 68-78. doi: 10.1001/jama.2020.7848. Erratum in: JAMA. 2020 Oct 13;324(14):1468. PMID: 32633800; PMCID: PMC7341181
- Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet, 2016; 388(10059): 2532-2561. doi: 10.1016/S0140-6736(16)31357-5. Epub 2016 Sep 8. Erratum in: Lancet. 2017 Feb 11;389(10069):602. PMID: 27616593.
- Giral P, Neumann A, Weill A, Coste J. Cardiovascular effect of discontinuing statins for primary prevention at the age of 75 years: a nationwide population-based cohort study in France. European Heart Journal. 2019; 40: 3516-3525. DOI: 10.1093/eurheartj/ehz485
- Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM, et al. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med. 2010; 152(8): 488-496, W174. Doi: 10.7326/0003-4819-152-8-201004200-00005.
- Dutch General Practioners Association (NHG). Practical guideline NHG-Standard CVRM, 2019.
- Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010; 376(9753): 1658-1669.
- Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. Journal of Clinical Lipidology, 2012; 6(3): 208-215.
- Shepherd J, Blauw GJ, Murphy MB, Bollen ELEM, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002; 360(9346): 1623-1630. DOI: 10.1016/s0140-6736(02)11600-x.
- NIH U.S. National Library of Medicine, ClinicalTrials.gov. A Clinical Trial of STAtin Therapy for Reducing Events in the Elderly (STAREE).
- University Medical Center Utrecht and ORTEC. U-prevent.
- Dutch Federation of Medical Specialists. Cardiovascular Risk Management (CVRM); table 1 risk categories, 2021.

