Accuracy of Post-Void Residual Urine Volume Measurement in Nigerian Men being evaluated for Prostate-related Lower Urinary Tract Symptoms: A Comparison of Ultrasonographic and Catheterization Methods
Augustine Obasi Ulebe, Timothy Uzoma Mbaeri, Joseph Amauzo Abiahu, Michael Echeta Aronu, Chinonso Odo, Anselm Okwudili Obi, Kingsley Chidi Oranusi, Jideoffor Chukwuma Orakwe, Alexander Maduaburochukwu Nwofor, Okechukwu Obiora Mbonu
Department of Surgery, Alex Ekwueme Federal University Teaching Hospital, Abakaliki, Nigeria.
Department of Surgery, Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria.
Radiology Department, Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria.
Received Date: 26/10/2021; Published Date: 26/11/2021
*Corresponding author: Augustine Obasi Ulebe, Department of Surgery, Alex Ekwueme Federal University Teaching Hospital, Abakaliki, Nigeria
Abstract
Background: Post-Void Residual urine volume (PVR) is one of the non-invasive tests that can predict voiding dysfunction. Measurement of PVR is commonly done by ultrasonographic and catheterization methods. Although catheterization method is regarded as the gold standard, it is invasive. Alternatively, the ultrasonographic method has been shown to be non-invasive but less accurate. The aim of this study is to determine the accuracy of ultrasonographic measurement of PVR using catheterization method as the gold standard.
Methods: Consecutive consenting male patients aged ≥ 40 years with prostate-related LUTS were assessed clinically followed by both ultrasonographic and catheter measurement of PVR. Spearman’s correlation and Bland-Altman plot were used to assess the correlation and agreement respectively between the 2 measurement methods. Data were analyzed using SPSS version 20.0, and P- Value < 0.05 was considered significant.
Results: The mean ultrasonographic -measured PVR (USSMPVR) and catheter-measured PVR (CMPVR) were 380.54 +24.21 ml and 399. +23.78 ml respectively. The mean difference between the 2 measurement methods was 17.85 + 37.82 ml (p<0.0001), with an accuracy of 46.0% recorded in ultrasonographic measurement of the PVR. A very strong correlation was noted between the CMPVR and the USSMPVR (Spearman r2 = 0.977, p<0.0001). The Bland-Altman plot showed greater variation and un-proportional bias between the catheterization and ultrasonographic methods despite high correlation with standard error of 0.016 (P-value = 0.152).
Conclusions: Despite the high positive correlation noted between the CMPVR and the USSMPVR, the study has shown that the ultrasonographic method is only 46% accurate compared to the gold standard which is the catheterization method.
Keywords: Bladder volume; Post-void residual; Catheterization; Ultrasonography; Prostate diseases
Abbreviations: BPH: Benign Prostatic Hyperplasia; BPE: Benign Prostatic Enlargement; BOO: Bladder outlet obstruction; LUTS: Lower Urinary Tract Symptoms; PVR: Post-Void Residual urine volume; USS: Ultrasound Scan/ Ultrasonographic; UTI: Urinary Tract Infection; IPSS: International Prostate Symptom Score; IBE: Incomplete Bladder Emptying; CMPVR: Catheter-measured Post-void residual urine volume; USSMPVR: Ultrasonographic-measured Post-void residual urine volume; PMBV: Pre-micturiction bladder volume; SPSS: Statistical Package for Social Sciences; PLESS: Proscar Long-Term Efficacy and Safety Study; VV: Voided volume; QoL: Quality of life.
Introduction
Prostate-related diseases with Lower Urinary Tract Symptoms (LUTS) are commoner among older men, and incidence increases with age [1]. These are often associated with voiding dysfunction, incomplete bladder emptying (IBE) and increased Post-Void Residual (PVR) urine volume. The urodynamic study is the gold standard for the diagnosis of voiding dysfunction but it is invasive. Post-void residual urine volume is one of the non-invasive tests that can predict voiding dysfunction. The PVR is defined as the volume of urine remaining in the bladder after an act of voiding [2].
There is still no consensus on the upper limit of PVR [3], the cut-off value for designating PVR as abnormal is arbitrary and usually ranges from 50 to 200ml [3]. Accurate PVR measurements are important for diagnosing voiding dysfunction and making clinical decisions regarding treatments of patients. Clinical measurement of PVR is commonly done by ultrasonographic and catheterization methods. The catheterization method serves both diagnostic and therapeutic purposes unlike ultrasonographic method that is only diagnostic relying on catheterization for therapeutic drainage of excessive residual urine as in cases of urinary retention. The catheterization method has been regarded as the gold-standard method for accurate measurement of PVR [2,4]. However, this method has major limitations of being invasive with increased risks of urethral trauma and urinary tract infection [2,4,5].
It is generally believed that urine volumes obtained by urethral catheterization are the most accurate for PVR measurement [2,4]. However, some authors reported that urethral catheterization may not be as accurate in determining PVR as is generally perceived [6]. To overcome some of the limitations associated with catheterization method, some authors have proposed ultrasonographic method as an alternative [7,8]. The ultrasonographic method is a reliable, noninvasive, safe, and quick means of estimating bladder volume as has been shown by some studies [8,9], but less accurate due to its inherent limitation of being operator dependent [2]. There is paucity of local studies on accuracy of PVR measurement in our environment, hence the need for this study with the aim to determine the accuracy of ultrasonographic measurement of PVR using catheterization method as the gold standard.
Methods
This hospital-based cross-sectional observational prospective study was conducted between May 2014 and April 2015 amongst all new consecutive male patients aged ≥ 40 years with prostate-related LUTS who presented to Urology Clinics of Department of Surgery, Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria.
Ethical approval was obtained from the institutional ethical review board and written informed consents obtained from all the participants.
Excluded from the study were patients with in-dwelling urethral catheter, suprapubic cystostomy, history of previous bladder surgeries, concomitant urethral stricture, and ultrasonographic evidence of bladder diverticulum. Patients were evaluated with a validated International Prostate Symptom Score (IPSS) questionnaire and a structured proforma containing demographic data, relevant history, clinical examination findings and investigation results.
All USS examinations were done with real-time ultrasound scanner (Aloka Prosound SSD- 3500SX TM) using a 3.5 mHz transducer ultrasound probe. To minimize inter-observer variability, the USS measurement of bladder volumes was done in the Radiology Department of the same hospital by a single Consultant Radiologist using specified guideline. Each patient was instructed to drink plenty of water until first intense urge to void was experienced. The pre-micturictional bladder volume (PMBV) was then measured by ultrasound scan for each patient and documented. Thereafter, each patient was asked to urinate directly into a calibrated measuring cylinder using a funnel to ensure complete collection and this was recorded as the Voided Volume (VV). Immediately after voiding, the PVR was also measured and recorded sonographically. With patient lying supine, the transducer was placed suprapubic ally to view maximum longitudinal section usually in the midline. Maintaining the same contact point, the transducer was then rotated 90o and angled up and down to find the largest transverse area. Ultrasound imaging was used to obtain sagittal and transverse images of the largest cross-sections of bladder visualized. The images were measured in three orthogonal directions: from the top to bottom of the bladder (H) and at 90° to this (D) in the sagittal plane and from left to right in the transverse plane (W) (Figure1).

Figure 1: Scan planes for ultrasound measurement of PVR.
The bladder volume was calculated using the formula, volume = H x D x W x k where k is correction coefficient. The PVR for each patient in this study was calculated using the Poston formula [10] with correction coefficient of 0.7 (i.e. H x D x W x 0.7).
Catheterization was done immediately after ultrasonographic measurement of PVR for each patient under strict aseptic condition using a well lubricated size 16Fr Silicone coated latex Foley’s catheter by in- and- out method. Complete drainage and emptying of the bladder were ensured by suprapubic compression of urinary bladder. A calibrated measuring cylinder with funnel was also used to collect and measure the volume of urine drained by catheterization after voiding with this recorded as the CMPVR. The CMPVR was then used as the gold standard for comparison with USSMPVR. Stop-watch was used to record the time interval between voiding and ultrasonographic measurements as well as between ultrasonographic measurement and catheterization for each patient. Prostate volume was also measured sonographically and recorded for each patient.
Data collected were analyzed with Statistical Package for Social Sciences (SPSS) version 20 (IBM; SPSS, Chicago, IL, USA). Results obtained were expressed using tables and charts where necessary. Simple frequencies were determined for age and descriptive statistics for the bladder volume measurements. Pearson’s correlation was used to assess the correlation between the 2 measurement methods. Bland-Altman plot was used to statistically assess the agreement between the ultrasonographic and catheterization methods of PVR measurement. P-value < 0.05 was considered to be statistically significant.
Results
A total of 100 men who met the inclusion criteria were recruited into the study. Their mean age was 71.02+ 9.10 years with the peak age range of 70.0 to 79.0 years (Figure 2).
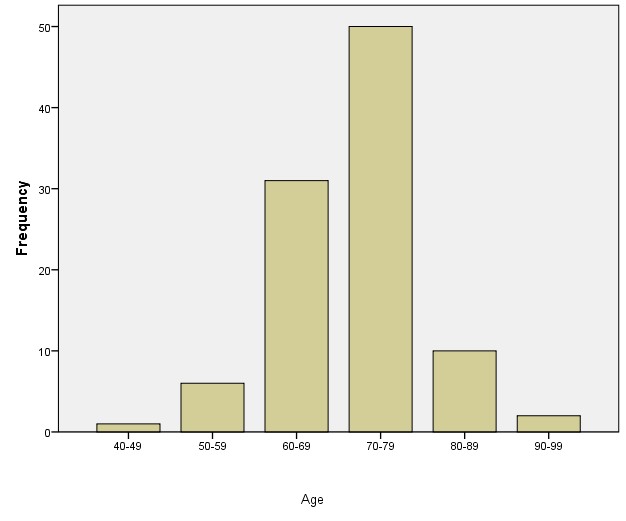
Figure 2: Age distribution of patients.
The duration of symptom ranged from 3.0 to 120.0 months with mean of 30.63 + 26.6 months. The mean IPSS mean was 20 .94+ 6.13 with the majority of the patients having IPSS in the range 16-20. Forty-three patients (43.0%) and 57 patients (57.0%) had moderate (IPSS=8-19) and severe LUTS (IPSS=20-35) respectively. None of the respondents had mild LUTS (IPSS=0-7). The QoL score ranged from 1.0 to 6.0 with mean of 4.80 + 1.08 with the majority (52.0%) of the patients having QoL score of 5.
The mean prostate volume and total serum PSA in this study were 116.49 + 75.19 ml and 55.44+47.11ng/dl respectively. The underlying causes of prostate-related bladder outlet obstruction (BOO)/LUTS was BPH in 56 patients (56.0%), adenocarcinoma in 33 patients (33.0%) and chronic prostatitis in 11 patients (11.0%).
The mean USSMPVR and CMPVR were 380.54 +24.21 ml and 399. +23.78 ml respectively with mean difference of 17.85 + 3.78 ml (p<0.0001). The mean time interval between voiding and USS measurement was 4.5 + 3.2 minutes (2.5-5.8minutes) while that between USS measurement and catheterization of patient was 5.5 + 2.2 minutes (3.5-7.5minutes). Hence, time interval between all the measurements was < 10 minutes (Table I).
Table 1: Demographic and Clinical Characteristics of the Patients.
Twenty three percent (23.0%) of patients had PVR ≤ 200 ml (the normal value for the study) while seventy-seven (77.0%) have abnormal PVR value >200ml (Figure 3).
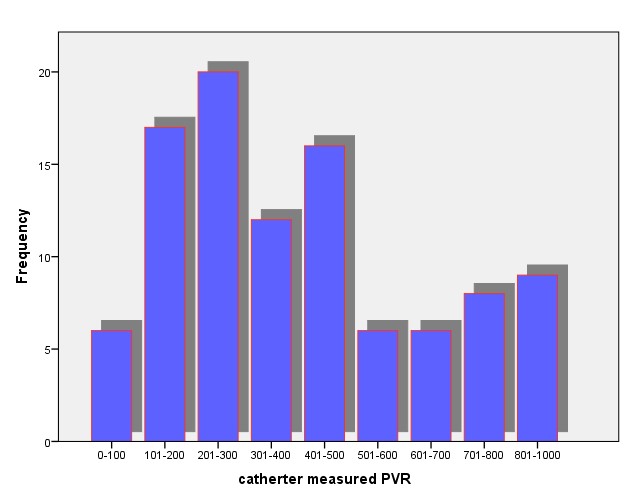
Figure 3: Distribution of Catheter-measured PVR.
Using the catheter-measured PVR as the gold-standard and limit of accuracy of -30 ml to + 30 ml, the ultrasonographic measurement of PVR was accurate in 46 measurements thereby giving accuracy rate of 46.0%. However, ultrasound over-estimated and under-estimated PVR in 12 (12.0%) patients (over-estimation group) and 42 (42.0%) patients (under-estimation group) respectively. None of the patients had the USSMPVR that was equal to CMPVR.
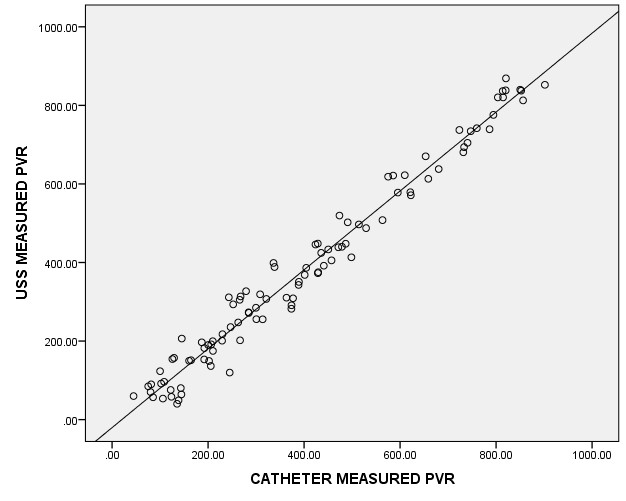
The Bland-Altman plot in assessing the agreement between the catheterization and the ultrasonographic methods showed a greater variation and un-proportional bias between the catheterization and ultrasonographic methods with standard error of 0.016 and P-value = 0.152 (Figure 4). There was a very strong correlation between the CMPVR and the USSMPVR with Spearman correlation coefficient r2 = 0.977 and p<0.0001(Figure 5).
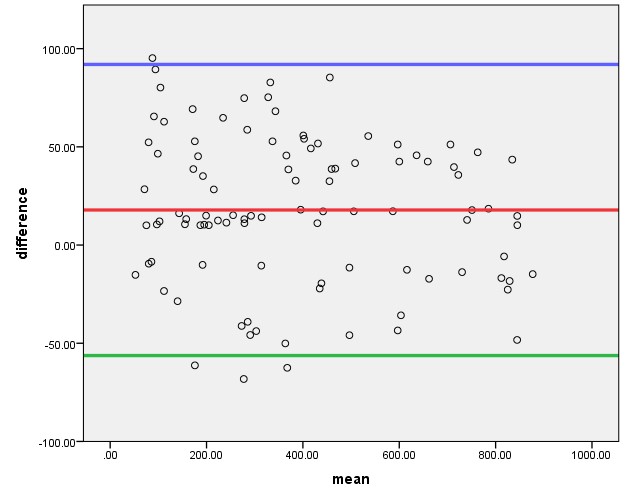
Figure 4: Bland-Altman Plot (Keys: Difference = difference between 2 measurement methods & Mean = mean of the 2 measurement methods for each patient; Red line = Mean diffrence, Blue line= Mean + 2SD & Green line = Mean -2SD).
Discussion
Prostate-related diseases with LUTS, voiding dysfunction, IBE and elevated PVR are commoner among older men, and incidence increases with age [1]. The mean age of patients in this study was 71.02+ 9.10 years. This is at variance with mean age noted in other studies [2,11,12]. In Iran, Simforoosh N et al [2] studied 324 men with persistent LUTS due to BPH and recorded a mean age of 61.5+ 8.32 years. Also, in Ilorin, Nigeria, Amole et al [11] who assessed 52 consecutive patients with BPH noted mean age of 64.98+7.57 years while in Egypt, Hassan et al [12] observed a mean age of 63.8+10.47 years. The possible explanation for the differences in mean age has to do with the composition of the study population. This present study included men with LUTS secondary to both BPH and prostate cancer. Available epidemiological data has shown the peak incidence of the prostate cancer to occur at a relatively higher age group compared to BPH [13]. With a significant proportion (33.00%) of patients having histopathologic diagnosis of prostate cancer in this study, it is not surprising that the mean age is similar to mean age noted by Ogunbiyi JO et al [13] in Ibadan, Nigeria amongst men with diagnosis of prostate cancer. On the other hand, the studies by Simforoosh N et al [2], Amole OA et al [11] and Hassan AA et al [12] share similar mean age because these 3-research works recruited only men with diagnosis of BPH in their study population. This explains the lower but similar mean ages seen in these studies [2,11,12] compared to higher mean age found in our study.
The mean IPSS noted in this study was 20.94+6.13 which falls into “severe” category. Hassan AA et al [12], in their study recorded mean IPSS of 16.18+ 8.65 which was categorized as “moderate”. The mean prostate volume in this study was 116.49 + 75.19ml with majority (91.0 %) of patients having prostate volume > 40ml while the mean PSA was 55.44+47.11ng/dl. These findings were in sharp contrast with the mean prostate volume of 54.66 + 21.52 ml and mean serum PSA of 1.74 + 0.85 ng/dl noted by Hassan AA et al [11] in their study. Hence, the patients in our study had more severe LUTS (as assessed by IPSS) with higher mean prostate volume and mean serum PSA values compared to those of patients in the study by Hassan AA et al [12].
From this observation, it may be suggested that these parameters (IPSS, Prostate volume, serum PSA) have clinical implications and may contribute to the significantly higher PVR values noted in our study with the mean USSMPVR and CMPVR of 380.54+ 24.21 ml and 399. +23.78 ml respectively. This finding was at variance with the lower mean PVR values noted by Hassan AA et al [12] in their study with the mean USSMPVR and CMPVR of 82.30+ 60.83 ml and 108.4 + 72.10 ml respectively. Several studies [14,15,16] have looked at the various risk factors for incomplete bladder emptying (IBE) with increased in PVR. The Proscar Long-Term Efficacy and Safety Study (PLESS) [14] have shown that the highest rates of IBE with urine retention and hence increase in PVR were recorded in men with a clinical diagnosis of Benign Prostatic Enlargement (BPE) having IPSS of ≥ 8.0, serum PSA >1.4ng/ml and prostate volume >40.0 ml. The Olmsted County Study of men with LUTS also found that prostate volumes > 30.0 ml, depressed peak urinary flow rate (<12ml/s) and advancing age as risk factors for IBE, urinary retention and increase in PVR [15].
Meigs et al [16] in their study of men with LUTS followed up for 8.0 years, have equally shown that men with a clinical diagnosis of BPE and IPSS ≥ 8.0 had the greatest incidence of IBE and increase in PVR.
In our study, all the patients had moderate-to-severe LUTS with IPSS ≥ 8.0. Also, majority (91.0%) of patients had prostate volume >40.0ml and all had a total serum PSA >1.4 ng/ml. Extrapolating findings in our study with the risk factors for IBE and increased PVR noted in these studies [14,15,16], it is not surprising that high mean PVR values were noted in our study. This corroborates the findings of significantly higher mean PVR in our study compared to lower mean PVR noted by Hassan AA et al [12] in their study considering multiple risk factors for elevated PVR noted amongst patients in this study.
The mean USSMPVR and CMPVR noted in our study were in discordance with the mean USSMPVR and CMPVR of 220.51 ml and 220.76 ml respectively noted by Amole OA et al [11]. Hassan AA et al [12] in their study in Egypt, recorded mean USSMPVR and mean CMPVR of 82.30+ 60.83 ml and 108.4+72.10 ml respectively. Young-Hyun et al [17] also recorded mean CMPVR of 265.10ml and mean USSM PVR of 239.ml. The values recorded by Young-Hyun et al [17] were somewhat lower than PVR values noted in our study but higher than the findings by Amole O A et al [11] and Hassan AA et al [12] in their respective studies.
Many authors [17,18] have expressed concern regarding short-comings of comparisons of results of research works involving ultrasonographic measurement of PVR. Various confounding factors affecting ultrasonographic measurement of PVR, including its operator dependence nature and the variations in research methodology approaches, make such comparison difficult. These confounding factors can be operator-related [12], instrument-related [17] or patient-related [19,21,22]. This proffers possible explanation for variations in values of PVR measurements obtained in this study compared with that noted in other studies [11,12,17].
The ultrasound probe used during examination play important role as depth of tissue penetration and image resolution/quality are both probe-dependent. For instance, all sonographic examinations in our study were done with real-time ultrasound scanner using a 3.5 mHz transducer ultrasound probe. Young-Hyun, et al [17] used 2.8mHz probe while both Simforoosh N, et al [2] and Hassan, et al [12] used 5mHz USS probe for their own study. Amole O A et al [11] however, used similar 3.5mHz USS probe but with different ultrasound machine design (Siemens Sonoline Sx scanner). These differences in the frequencies of ultrasound probes used in different studies may account for the differences noted in these research results and hence the difficulty in comparing different studies. Effort should made to harmonize and standardize ultrasonographic measurement of PVR so that evidence-based comparison of research works will be possible.
In this study, it was found that there was a very strong positive correlation between the CMPVR and USSMPVR (r2 = 0.977 and p<0.0001). This is similar to work by Amole O A et al [11] who also found a very strong positive correlation between the CMPVR and the USSMPVR which is statistically significant (r2=0.982, p<0.0001). Other studies including Young H P et al [17] (r2 = 0.95, p< 0.001), Cardenas DD et al [18] (r2=0.80, p< 0.001), Lertbunnaphong et al [23] (r2=0.93, p<0.001), Luk JKH et al [24] (r2= 0.93, p<0.0001), and Ghadeer ASM et al [25] (r2=0.79, p<0.001) have also noted positive correlation between the CMPVR and the USSMPVR. However, the study done by Simforoosh N et al [2] failed to demonstrate any correlation between the CMPVR and the USSMPVR for any of 11 formulas used in ultrasonographic measurement of PVR. The authors concluded that ultrasound scan cannot rapidly measure bladder volumes accurately till date and that catheterization still remain the most accurate method to assess PVR in clinical practice [2].
In this study, the mean difference between the USS-measured PVR and the catheter-measured PVR (i.e., catheter-measured PVR minus USS-measured PVR) was 17.85 + 3.78 ml which is statistically significant (p<0.0001). In Ilorin, Amole OA et al [11] recorded mean difference of 0.25ml which is not statistically significant (p<0.01) while Hassan AA et al [12] in their study in Egypt noted a mean difference of 26.10 ml which is statistically significant (p<0.0001). Ghadeer ASM et al [25] in assessing the accuracy of bladder scanning in measurement of PVR noted a high correlation between USSMPVR and CMPVR with a mean difference of 12.9ml between the 2 methods.
Using the CMPVR as the gold-standard with limit of accuracy of -30 ml to + 30 ml, ultrasonographic measurement of PVR was accurate in 46 patients while over-estimating and under-estimating PVR in 12 (12.0%) and 42 (42.0%) patients respectively. None of the patients had USSMPVR that was equal to CMPVR. Therefore, an accuracy rate of 46.0% was noted in our study. This was at variance with the findings in these studies [2,23,25]. Ghadeer ASM, et al [25] in their recruiting 96.0 patients, noted that 62.0 patients had accurate USS measurement of PVR with an accuracy rate of 64.6% while over-estimating and under-estimating PVR in 8.0 (8.3%) and 26.0 (27.1%) of patients respectively. Simforoosh N et al [2] in assessing the accuracy of PVR measurement in 324 men using 11 different formulae for ultrasonographic calculation of PVR noted accuracy rates ranging from 0.33+ 0.23 to 1.51+ 0.71 depending on formula applied with an accuracy rate of 0.53+0.36 (53.0%) recorded using Poston formula. The accuracy rate of 53.0% noted by Simforoosh N et al using Poston formula is lower than 64.6% noted by Ghadeer ASM et al [24] but higher than 42.0% noted in our study. Lertbunnaphong T, et al [23] in assessing the correlation between the CMPVR and USSMPVR in women after hysterectomy, recorded an accuracy rate of 87.0% in which the USSMPVR is accurate in 40 out of total number of 46 patients recruited into their study. This accuracy rate of 87.0% recorded by Lertbunnaphong T et al [23] is much higher than that note in our study and other studies [2,25]. These differences in accuracy rate noted may be related to differences in patient characteristics and methodology approaches as well as observer dependence nature of ultrasonographic measurements.
Young HP et al [17], in assessing the accuracy of PVR measurement, noted that bladder scan without real-time pre-scan imaging (RPI) tends to overestimate the true PVR measurement by a mean Percent of Differences of Volume (PDV) of 16.3% while bladder scanner with RPI tends to underestimate true PVR measurements by a mean PDV of -14.1%. This study [17] has demonstrated the effect of instrument-related factors in determining accuracy of PVR measurements.
However, all the studies had proven that ultrasonographic measurements is not 100.0% accurate in PVR measurement and this should be taken into account when using USSMPVR in clinical evaluation of patients with voiding dysfunction.
The ultrasound overestimated PVR in 12 (12.0%) patients. A common cause of over-estimation of PVR measurement include the presence of predominantly echo-free pelvic simple cysts with echogenicity similar to that of water which can lead to falsely elevated values in PVR measurement [21,22]. Another possible apparent cause of USS over-estimation is error in catheter measurement by inadequate and incomplete catheter drainage of post-void residual urine. Although some authors regard in-and-out bladder catheterization as the gold standard for an accurate PVR measurement [2], others have reported urethral catheterization to be not as accurate in determining PVR volume as is generally perceived [6]. The surplus length of the catheter during use and the position of the eyelets far from the bladder neck resulting in incomplete emptying are possible reasons given by authors for catheter under-estimation of PVR [8]. However, during this study, complete drainage and emptying of the bladder was ensured by pulling the catheter out slowly stopping anytime the urine flows while maintaining suprapubic compression of the urinary bladder.
Ultrasound was noted to under-estimate PVR in significant proportion (42.0%) of patients in this study. Some investigators have given the possible reasons for USS under-estimation of PVR to include irregular shape of the bladder, continued bladder filling during the delay before catheterization, and failure of the scan to include all parts of the bladder because of large bladder volume [19,20]. To correct and account for the impact of variation in bladder shape on bladder volume measurement, Bih et al [19] recommended multiplying the measured bladder dimensions with a correction coefficient “k” to reduce errors in measurements due to variation in bladder shape with filling. This was applied in this study with correction coefficient of 0.7 using the conventional formula proposed by Poston et al [10]. Another possible explanation of ultrasonographic under-estimation of PVR in this study may be due to further accumulation of extra urine during the time interval between USS measurement of PVR and catheterization. This may erroneously lead to USS under-estimation of PVR as a result of extra volume of urine added to the bladder during the time lag between measurements thereby increasing CMPVR above USSMPVR. The mean time interval between voiding and ultrasonographic measurement in this study was 4.5 + 3.2 minutes while that between USS measurement and catheterization of patient was 5.5 + 2.2 minutes. Different studies recorded various mean time intervals between measurements ranging from 1.5-2.5 minutes [2], 5.0 minutes [24], and 10.0 minutes [25]. Our study tried to reduce timing-related error by limiting the time interval between ultrasonographic measurement and catheterization to < 10.0 minutes.
There is no unified and standardized time interval between these measurements available in medical literature. Different authors used different time interval based on prevailing circumstances. However, the most important thing is to try and limit this time interval so as to reduce to barest minimum the error that may arise from such delays in measurement.
Interestingly, none of the patients had CMPVR that was equal to USSMPVR in our study. This is similar to findings of other studies that equally found that none of their patients had CMPVR that was equal to USSMPVR [11,12,17,18,23].
This study was also able to demonstrate from Bland-Altman plot [26] that despite the high positive correlation (r2 = 0.977, p<0.0001) between the CMPVR and USSMPVR, there were a greater variation and un-proportional bias between the catheterization and ultrasonographic methods, though this is not statistically significant (p > 0.05). This is in keeping with the findings of Ghadeer ASM et al [25] who also demonstrated similarly on Bland-Altman plot that despite the high positive correlation (r2=0.79, p<0.001) between CMPVR and USSMPVR, there were a statistically insignificant variation and un-proportional bias between the 2 measurement methods.
The low accuracy rate of 46.0% noted in our study despite high correlation between the CMPVR and USSMPVR with the Bland-Altman plot demonstrating a greater variation and un-proportional bias between the 2 methods, has shown the Bland-Altman plot to be a better tool for assessing agreement/accuracy between 2 clinical measurements than correlation study.
The finding of high positive correlation between 2 measurement methods does not necessarily mean high degree of accuracy of one measurement method when compared with the gold standard method. Extra effort should be made to determine the agreement between the 2 measurement methods using the Bland-Altman plot analysis in addition to assessing the accuracy rate and the correlation between the 2 methods.
Hence, the ideal approach to establishing superiority of one measurement method in terms of accuracy over another should include triple statistical analysis of the correlation, Bland-Altman plot analysis and determination of the accuracy rate. Different authors used different statistical analysis approaches in their respective studies. Some research works determined the correlation between CMPVR and USSMPVR without calculating the accuracy rate and Bland-Altman plot analysis. [11,18]. While others assessed the correlation between 2 measurement methods and the accuracy rate calculated without Bland-Altman plot analysis [2,23]. Also, some studies assessed only the accuracy rate without determining correlation and Bland-Altman plot analysis [7,8,17,20]. Few studies including our study and the one done by Ghadeer ASM, et al [25] employed the triple statistical analytical approach by determining the correlation and Bland-Altman plot analysis between the 2 measurement methods in addition to assessing the accuracy rate.
With standardization of its measurements, the PVR measurements may emerge in the near future as one of the most useful non-invasive tests in evaluation of patients with prostate-related LUTS.
One major limitation of this study is that catheterization method of PVR measurement is invasive with increased risks of urethral trauma and urinary tract infection. Effort was made to limit these risks by using appropriately sized urethral catheter, passed under strict asepsis with adequate lubrication.
To enhance external validity/generalizability of this study, similar larger sample-sized studies are recommended in our environment in order to extrapolate the findings of our study to a larger population.
Conclusion
Despite the high positive correlation noted between the CMPVR and the USSMPVR, the study has shown that the ultrasonographic method is only 46% accurate compared to the gold standard which is the catheterization method. The USS method can under-estimate or over-estimate the PVR depending on various patient-related, instrument-related and operator-related confounding factors. Whenever the exact value of PVR measurement is needed for patient care, the more accurate catheterization method should be used. However, this approach should delicately balance the benefits of accuracy in measurement with the risks associated with invasive nature of catheterization method.
Declarations
Ethics approval and consent to participate:
Ethical approval was obtained from the Health Research Ethics Committee (HREC) of Nnamdi Azikiwe University Teaching Hospital, Nnewi, Anambra State, Nigeria (Reference number: NAUTH/CS/66/VOL5/57).
A written informed consent was obtained from all the participants. Participation was voluntary, and confidentiality was maintained throughout the study.
Consent for publication: Not applicable.
Availability of data and materials: primary data is available in a secured storage.
Competing interest: All authors have no competing interest.
Funding: This study received no external funding.
Authors’ contributions:
AOU, TUM and MEA participated in conceptualization of the research work, carry out acquisition, analysis, interpretation of data and drafted the first manuscript.
JCO, AMN, and OOM reviewed the data analysis and contributed to subsequent drafts of manuscript.
JAA, CO, AOO and KCO participated in literature search, review analysis and critically revised the manuscript.
All authors read and approved the final manuscript.
Acknowledgement: None.
References
- Borrie MJ, Campbell K, Arcese ZA, Bray J, Hart P, Labate T, et al. Urinary retention in patients in a geriatric rehabilitation unit: prevalence, risk factors, and validity of bladder scan evaluation. Rehabil Nurs 2001; 26: 187-191.
- Simforoosh N, Dadkhah F, Hosseini SY, et al. Accuracy of residual urine measurement in men: Comparison between real-time ultrasonography and catheterization. J Urol 1997; 158: 59-61.
- Urinary incontinence in adults: acute and chronic management, clinical practice guideline. Number 2 (1996 update). Agency for Health Care Policy and Research (AHCPR) Publication No. 96- 0682, 1996.
- Mainprize TC, Drutz HP. Accuracy of total bladder volume and residual urine measurements: comparison between real-time ultrasonography and catheterization. Am J Obstet Gynecol 1989; 160:1013-1016.
- Purkiss SF. Assessment of residual urine in men following catheterization. Br J Urol 1990; 66: 279-280.
- Stoller ML, Millard RJ. The accuracy of a catheterization residual urine. J Urol 1989; 141(1): 15–16.
- Griffiths CJ, Murray A, Ramsden PD. Accuracy and repeatability of bladder volume measurement using ultrasonic imaging. J Urol 1986; 136: 808-812.
- Byun SS, Kim HH, Lee E. Accuracy of bladder volume determinations by ultrasonography: Are they accurate over entire bladder volume range? Urology 2003; 62: 656-660.
- Barrington J, Bowen-Simpkins P. A comparison between portable ultrasound and urethral catheterization in the measurement of female bladder volume. J ObstGynaecol.1996; 16: 50-51.
- Poston GJ, Joseph AEA, and Riddle PT: The accuracy of ultrasound in measurement of changes in bladder volume. Br J Urol.1983; 55: 361-363.
- Amole OA, Sulyman AK, Oyejola BA. Sonographic assessment of post-void residual urine volumes in patients with benign prostatic hyperplasia. J Natl Med Assosc.2004; 96: 234-239.
- Hassan AA, Housseini MA, Mahmoud HS, Mohamed BI, Mostafa AS. The reliability and reproducibility of ultrasonography for measuring the residual urine volume in men with lower urinary tract symptoms. Arab Journal of Urol 2014; 12: 285-289.
- Ogunbiyi JO, Shittu OB. Increased incidence of prostate cancer in Nigerians. J Natl Med Assoc. 1999; 91: 159-164.
- Kaplan S, Garvin D, Gilhooly P. Impact of baseline symptom severity on future risk of benign prostatic hyperplasia related outcomes and longterm response to finasteride. The PLESS Study Group.Urology. 2000; 56: 610-616.
- McConnell JD, Barry MJ, Bruskewitz RC. Benign prostatic hyperplasia: diagnosis and treatment. Agency for Health Care Policy and Research, ClinPract Guide Quick Ref Guide Clin.1994; 8: 1-17.
- Meigs JB, Barry MJ, Giovannucci E, Rimm EB, Stampfer MJ, Kawachi I. Incidence rates and risk factors for acute urinary retention: the health professionals follow-up study. J Urol. 1999;162: 376-382.
- Young HP, Ja-Hyeon K, Seung-June O. Accuracy of Post-void Residual Urine Volume Measurement Using a Portable Ultrasound Bladder Scanner with Real-time Pre-scan Imaging. Neurourol Urodynam 2011; 30: 335-338.
- Cardenas DD, Kelly E, Krieger JN. Residual urine volumes in patients with spinal cord injury: Measurement with a portable ultrasound instrument. Arch Phys Med Rehabil 1988; 69: 514-516.
- Bih LI, Ho CC, Tsai SJ, Lai YC, Chow W. Bladder shape impact on the accuracy of ultrasonic estimation of bladder volume. Arch Phys Med Rehabil 1998; 79: 1553–1556.
- Coombes GM, Millard RJ. The accuracy of portable ultrasound scanning in the measurement of residual urine volume. J Urol 1994; 152: 83-85.
- Cooperberg MR, Chambers SK, Rutherford TJ, Foster HE Jr. Cystic pelvic pathology presenting as falsely elevated post-void residual urine measured by portable ultrasound bladder scanning: report of 3 cases and review of the literature. Urology 2000; 55(4): 590-592.
- Dunn IB, Palmer M. Erroneous diagnosis of chronic urinary retention in three women with pelvic cysts. Scand J UrolNephrol 2000; 34(6): 381-382.
- Lertbunnaphong T, Inthasorn P, Boriboonhirunsarn D, Chuchotirot M, Russameecharoen K, Phattanachidakun B. Transabdominal Ultrasound in the Assessment of Post-void Residual Urine Volume in Patients after Hysterectomy. J Med Assoc Thai 2006; 89(Suppl.4): S152-S157.
- Luk JKH, Tam MW, Ho MCS, Chan FHW. Managing older patients with urinary retention in the Continence Clinic. Hong Kong Med J 2003; 9: 15-19.
- Ghadeer ASM, Annick L, Craig EC. Accuracy of Bladder Scanning in the Assessment of Postvoid Residual Volume. Am J Obstet Gynecol, 2010;175(1): 10–17.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307-310.

