Role of Bamlanivimab Plus Etesevimab in Covid-19 Therapy
Aditya Rai1, Jaysheela J*, Somasundaram G*, Prasad DN*
Department of Pharmacology, Sri Lakshmi Narayana Institute of Medical Sciences, Pondicherry, India
Department of Pharmacology, Shivalik College of Pharmacy, India
Received Date: 18/10/2021; Published Date: 15/11/2021
*Corresponding author: Dr. J. Jaysheela, Assistant Professor, Department of Pharmacology, Sri Lakshmi Narayana Institute of Medical Sciences, India.
Dr. G. Somasundaram, HOD, Department of Pharmacology, Sri Lakshmi Narayana Institute of Medical Sciences, India.
Dr. D.N.Prasad, Principal, Department of Pharmacology, Shivalik College of Pharmacy, Nangal, India.
Adwiza Rai, 4th Year MBBS, Sri Lakshmi Narayana Institute of Medical Sciences, pondicherry, India.
Abstract
Covid-19, which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread around the world and continues to represent a major health risk. Covid-19 can be mild to severe, and a susceptible subset of individuals has been found to have a high frequency of sickness and mortality [1-4]. Patients over the age of 65, as well as those with chronic health complications such as cardiovascular disease, cancer, diabetes, lung disease, and obesity, have a higher chance of dying from Covid-19 [5,6]. Unlike vaccine-induced immunity, which takes years to develop, neutralizing monoclonal antibody therapy delivers rapid, passive protection, which may help to slow disease progression and consequences.
Keywords: COVID-19; Bamlanivimab; Etesevimab; Monoclonal Antibody; Spike proteins; BLAZE-1 Trial
Introduction
Patients with Covid-19 have been treated with a variety of therapies [7-9], including convalescent plasma [10] and immunomodulators [11,12], but the outcomes have been variable. The Food and Drug Administration (FDA) issued emergency use authorization for the SARS-CoV-2 spike protein-targeting messenger RNA vaccines BNT162b2 [13] and mRNA-1273 [14] in December 2020. There is still a need for medications to treat patients who have symptomatic Covid-19 prior to vaccination or who have breakthrough infection. Immediate passive humoral immunotherapy with neutralizing monoclonal antibodies could be used to avoid Covid-19-related hospitalization and death [15].
In the United States and China, Bamlanivimab and Etesevimab were isolated from convalescent plasma collected from patients with Covid-19 [16,17]. The surface spike glycoprotein of SARS-CoV-2, which mediates viral entry into host cells, is the target of these potent neutralizing monoclonal antibodies [18,19]. Bamlanivimab was developed by Eli Lilly after it was discovered by researchers at AbCellera Biologics and the National Institute of Allergy and Infectious Diseases' Vaccine Research Center. Eli Lilly, Junshi Biosciences, and the Chinese Academy of Sciences' Institute of Microbiology collaborated on the development of Etesevimab.
Both monotherapy with bamlanivimab and combination therapy with bamlanivimab and etesevimab were effective in reducing the risk of Covid-19–related hospitalisation and progression to severe disease in the phase 2 and early portion of the phase 3 Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) clinical trial. As a result, the FDA granted bamlanivimabmonotherapy emergency use authorization in November 2020 [20], however this authorization was later rescinded. In February 2021, the FDA awarded emergency use authorization status to bamlanivimab and etesevimab when used jointly [21].
Structure
Bamlanivimab (LY-CoV555) is a synthetic monoclonal antibody (mAb) generated from one of the earliest COVID-19-positive blood samples in the United States [22]. It's a neutralising IgG1 mAb that targets the SARS-CoV-2 spike (S) protein, which has been shown to prevent viral entrance into human cells.
The Fc region of Bamlanivimab is unaltered and contains two identical light chains of 214 amino acids and two identical heavy chains of 455 amino acids apiece (Figure 1). Chinese Hamster Ovary (CHO) cells are used to synthesize bamlanivimab [23].
Figure 1: Structure of Bamlanivimab [27].
Etesevimab (LY-CoV016) is a recombinant monoclonal antibody that targets the binding domain of the SARS-CoV-2 surface spike protein receptor. LY-CoV016 binds a broad epitope that overlaps the ACE2-binding surface using both its heavy and light chains (Figure 2).
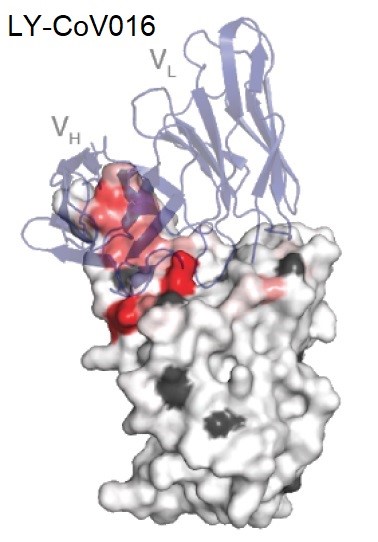
Figure 2: Structure of LY-CoV016 showing the heavy and light chain domains VH and VL [27].
Mechanism of Action
Many glycosylated S proteins coat the surface of SARS-CoV-2, allowing the virus to bind to the host cell's ACE2 (Angiotensin-converting enzyme-2 receptor) and enter the cell. S1 and S2 are the two subunits of the S protein. NTD (N-terminal domain) and RBD make up the S1 subunit (receptor-binding domain). The RBD of the S1 subunit interacts to the ACE2 of the host cell (Figure 3). The RBD is one of the most common targets for neutralizing monoclonal antibodies [30].

Administration
The authorized dose in adults and pediatric patients (12 years age and above; and weighing 40 kg or more) is 700 mg of bamlanivimab and 1400 mg etesevimab. The combination product comes in a package with the following [30].
- Bamlanivimab (700 mg) - 1 vial
- Etesevimab (700 mg) - 2 vials
- Sterile prefilled infusion bag - 0.9% sodium chloride (50 mL, 100 mL, 150 mL, or 250 mL)
The package should be stored in the refrigerator. The vials must be warmed to room temperature for 20 minutes before preparation without being exposed to direct heat. Withdraw 20 ml and 40 ml respectively of bamlanivimab and etesevimab from vials, then inject into a prefilled infusion bag. The preparation should not be shaken; instead, gently invert the infusion bag ten times to mix. The prepared solution can stay at room temperature for 7 hours, including the administration time, and it can be kept in the refrigerator for 24 hours. If refrigerated, the solution must be warmed to room temperature for 20 minutes before use [24,30].
The compatibility of the combination product with any other medications and IV solutions other than 0.9% sodium chloride is not known. So, it is advisable not to administer the prepared solution with any other medication. After the infusion is complete, flush the tubing with 0.9% sodium chloride to ensure the correct total dose is given [30].
Blaze-1Trial (Phase 3)
Trial Design: In this ongoing phase 2–3, randomized, double-blind, placebo-controlled, single-dose trial conducted in the United States, all the patients had recently received a diagnosis of mild or moderate Covid-19 in an outpatient setting. The patients presented with mild or moderate Covid-19 within 3 days after they had tested positive for SARS-CoV-2 by means of either direct antigen or nucleic acid identification [31].
The first patient was enrolled on September 4, 2020, and the last patient was enrolled on December 8, 2020. The patients received a single intravenous infusion consisting of either a combination of 2800 mg of bamlanivimab and 2800 mg of etesevimab or placebo over a period of 1 hour [31].
Patients: Ambulatory patients who were 12 to 17 years of age and who had at least one of the following risk factors at the time of screening were included in the trial: a BMI in at least the 85th percentile for age and sex, according to CDC growth charts [25]; sickle cell disease; congenital or acquired heart disease; neurodevelopmental disorders such as cerebral palsy; dependence on a medical-related mechanical device or procedure such as tracheostomy, gastrostomy, or positive-pressure ventilation (not related to Covid-19); asthma, a reactive airway, or another chronic respiratory disease; type 1 or type 2 diabetes mellitus; and an immunocompromised condition or receipt of an immunosuppressive treatment. Ambulatory patients who were at least 18 years of age and who presented with at least one of the following risk factors were also included: age of at least 65 years, a BMI of at least 35, chronic kidney disease, diabetes mellitus type 1 or type 2, immunosuppressive disease or receipt of immunosuppressive treatment, and an age of at least 55 years with cardiovascular disease, hypertension, or chronic obstructive pulmonary disease or another chronic respiratory disease.
For both adolescents and adults, mild or moderate Covid-19 was defined according to FDA guidance [26] and included the following eight symptoms: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, and shortness of breath with exertion. Notable exclusion criteria included a peripheral oxygen saturation of 93% or less while breathing ambient air, a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen of less than 300, a respiratory rate of at least 30 breaths per minute, and a heart rate of 125 or more beats per minute [31].
Outcomes: The primary outcome was the overall clinical status of the patients, defined as Covid-19–related hospitalization (acute care for ≥24 hours) or death from any cause by day 29. Key secondary outcomes were the change from baseline to day 7 in the SARS-CoV-2 viral load and a persistently high SARS-CoV-2 viral load on day 7 (defined as a log viral load >5.27, corresponding to a mean PCR cycle-threshold [Ct] value of <27.5). This threshold, which was identified in a post hoc analysis from the phase 2 part of the BLAZE-1 trial, was prespecified for the current analysis [31].
Other key secondary outcomes were a composite of a Covid-19–related hospitalization, a visit to an emergency department, or death from any cause by day 29 and the time to sustained patient-reported resolution of symptoms. Resolution was defined as an absence of all eight Covid-19–related symptoms except for mild cough or as an absence of fatigue for two consecutive assessments. Additional secondary outcomes were a reduction in the SARS-CoV-2 viral load from baseline to days 3 and 5, the time to viral clearance, the area under the response–time curve for the viral load through day 7, the time to a reduction and resolution of symptoms and safety [31].
Statistical Analysis
Primary Outcome: A total 11 of 518 patients (2.1%) in the bamlanivimab–etesevimab group, as compared with 36 of 517 patients (7.0%) in the placebo group, had a Covid-19–related hospitalization (defined as acute care for ≥24 hours) or death from any cause by day 29 (absolute risk difference, −4.8 percentage points; 95% confidence interval [CI], −7.4 to −2.3; relative risk difference, 70%; P<0.001). By day 29, none of the patients who received bamlanivimab plus etesevimab had died, and 10 of the 517 patients who received placebo had died (Figure 4). Of these 10 deaths, 9 were deemed to be Covid-19–related by trial staff who were unaware of the trial-group assignments [31].
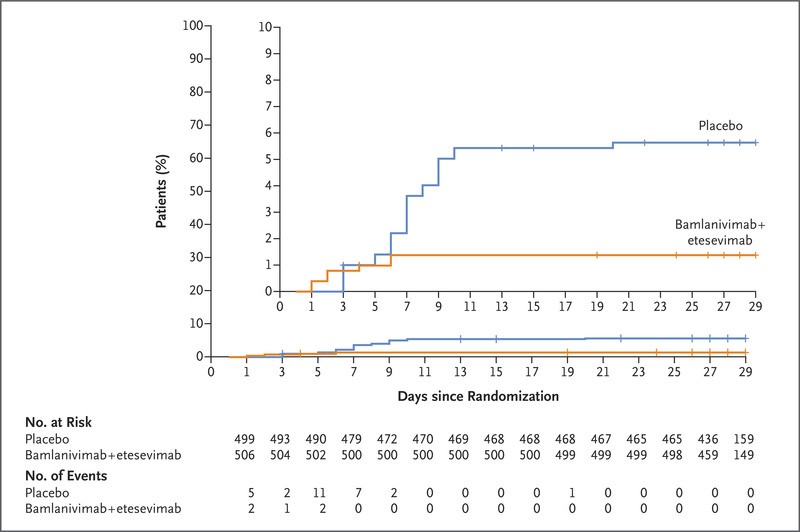
Figure 4: Time to Hospitalization among High-Risk Patients Who Received Bamlanivimab–Etesevimab or Placebo [31].
Key Secondary Outcomes: The mean reduction in the viral load from baseline to day 7 was approximately 16 times as high in patients who received bamlanivimab plus etesevimab as in those who received placebo (difference from placebo in the decrease from baseline, −1.20; 95% CI, −1.46 to −0.94; P<0.001). The percentage of patients with a persistently high viral load on day 7 was 9.8% (50 of 508 patients) in the bamlanivimab–etesevimab group, as compared with 29.5% (147 of 499 patients) in the placebo group (difference, −19.6 percentage points; 95% CI, −24.4 to −14.9; P<0.001) (Figure 5). Twelve of 518 patients (2.3%) who received bamlanivimab plus etesevimab had a Covid-19–related hospitalization, an emergency department visit, or death from any cause by day 29, as compared with 37 of 517 patients (7.2%) who received placebo (difference from placebo in the decrease from baseline, −4.8 percentage points; 95% CI, −7.4 to −2.3; P<0.001) [31].
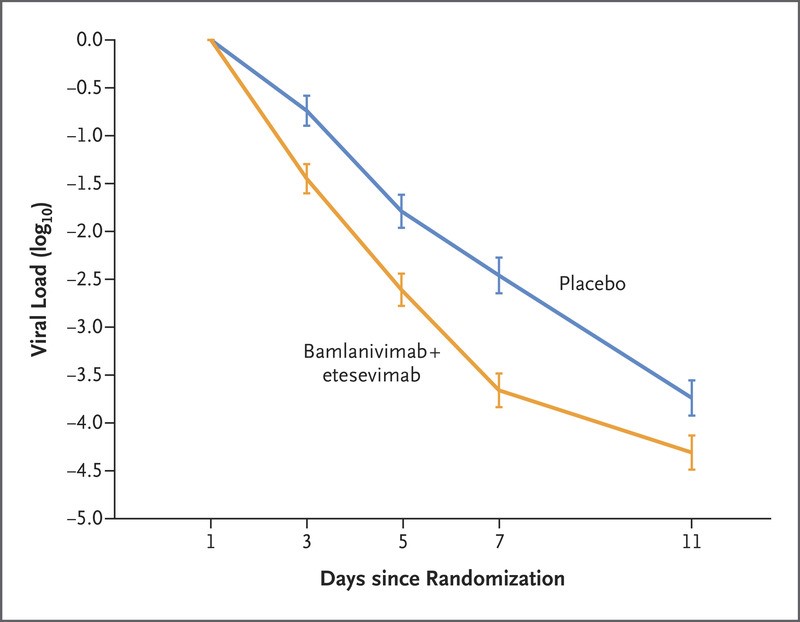
Figure 5: Effect of Bamlanivimab–Etesevimab on Viral Load [31].
Safety
Serious adverse events occurred in 7 of 518 patients (1.4%) in the bamlanivimab–etesevimab group and in 5 of 517 patients (1.0%) in the placebo group. Adverse events that occurred after the infusion was initiated were reported in 69 of 518 patients (13.3%) in the bamlanivimab–etesevimab group and in 60 of 517 patients (11.6%) in the placebo group (Table 1). In both groups, the most common adverse events were nausea, rash, dizziness, diarrhea, and hypertension [31].
Table 1: Adverse Events [31].

Toxicity: Clinical investigations with doses up to 7000 mg of bamlanivimab (10 times the FDA approved dose) or 7000 mg of etesevimab (5 times the FDA recommended dose) found no evidence of dose-limiting harm. In the event of a bamlanivimab and etesevimab overdose, supportive measures such as vitals and clinical status monitoring are used. For the time being, there is no specific antidote for an overdose [30].
Conclusion
The results suggest that early intervention with bamlanivimab with etesevimab was successful in reducing the occurrence of hospitalization. By day 29, the patients who got bamlanivimab + etesevimab had a 4.8 percentage point lower risk of Covid-19-related hospitalizations or mortality from any cause than those who received placebo [31].
There were no deaths among patients who got bamlanivimab plus etesevimab, but 10 deaths were reported among those who received placebo, 9 of which were deemed to be Covid-19–related by the investigators. Bamlanivimab plus etesevimab led in a rapid remission of symptoms within 4 days after starting therapy, in addition to lower rates of hospitalization and death.Bamlanivimab with etesevimab has an acceptable safety profile for usage in high-risk populations [31].
The administration of monoclonal antibody treatment still faces significant logistical obstacles. The therapeutic administration of bamlanivimabmonotherapy has shown the obstacles that already overburdened health care facilities face, such as the lack of space, personnel, and resources required to give medication [31].
While society works to stop the pandemic with widespread vaccination programs and efforts to acquire herd immunity, antibody therapy offers a viable treatment option to minimize the frequency of sickness and mortality among vulnerable patients.
Acknowledgement
I would like to thank Dr. Somusundaram and Dr. Jaysheela for all of their help and guidance. Without their assistance, writing this review would’ve been a far more difficult task than it was. The BLAZE-1 trials are a very vital part of the medical establishment’s struggle against the Covid-19 pandemic and I would like to thank the researchers and physicians who are doing their best to find ways of effectively treating an unforgiving disease. I would like to thank Dougan M, Nirula A, Azizad M, et al from the New England Journal of Medicine who have provided a vast amount of data from the BLAZE-1 trials and have kept everyone appraised of the progress being made.
References
- Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 2021; 384: 229-237.
- Cates J, Lucero-Obusan C, Dahl RM, et al. Risk for in-hospital complications associated with COVID-19 and influenza - Veterans Health Administration, United States, October 1, 2018–May 31, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1528-1534.
- Ejaz H, Alsrhani A, Zafar A, et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health 2020; 13: 1833-1839.
- Piroth L, Cottenet J, Mariet A-S, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med 2021; 9: 251-259.
- Chidambaram V, Tun NL, Haque WZ, et al. Factors associated with disease severity and mortality among patients with COVID-19: a systematic review and meta-analysis. PLoS One 2020; 15(11): e0241541-e0241541
- Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and cancer: a comprehensive review. CurrOncol Rep 2020; 22: 53.
- Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without Azithromycin in mild-to-moderate Covid-19. N Engl J Med 2020; 383: 2041-2052.
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med 2020; 383: 1813-1826.
- The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384: 693-704.
- Joyner MJ, Senefeld JW, Klassen SA, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience 2020.
- Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021; 384: 20-30.
- Roche. Roche provides an update on the phase III COVACTA Trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia, 2020.
- FDA takes key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine. News release of the Food and Drug Administration Silver Spring, MD, 2020.
- FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine. News release of the Food and Drug Administration Silver Spring, MD, 2020.
- Marston HD, Paules CI, Fauci AS. Monoclonal antibodies for emerging infectious diseases — borrowing from history. N Engl J Med 2018; 378: 1469-1472.
- Jones BE, Brown-Augsburger PL, Corbett KS, et al. LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection, 2020.
- Shi R, Shan C, Duan X, et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020; 584: 120-124.
- Benton DJ, Wrobel AG, Xu P, et al. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature 2020; 588: 327-330.
- Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003; 426: 450-454.
- Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. News release of the Food and Drug Administration, Silver Spring, MD, 2020.
- Coronavirus (COVID-19) Update: FDA authorizes monoclonal antibodies for treatment of COVID-19. News release of the Food and Drug Administration, Silver Spring, MD, 2021.
- Eli Lilly: Initial LY-CoV555 statement.
- FDA Emergency Use Authorization Fact Sheet: Bamlanivimab
- An EUA for bamlanivimab and etesevimab for COVID-19. Med Lett Drugs Ther. 2021; 63(1621): 49-50.
- Centers for Disease Control and Prevention. Clinical growth charts.
- Food and Drug Administration. COVID-19: developing drugs and biological products for treatment or prevention, 2020.
- A Study of LY3819253 (LY-CoV555) and LY3832479 (LY-CoV016) in Participants with Mild to Moderate COVID-19 Illness (BLAZE-1). ClinicalTrials.gov Identifier: NCT04427501
- Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. Tyler N. Starr et al. 2020.11.30.405472.
- The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Bryan E. Jones et al. Science Translational Medicine, 13(593).
- Balasundaram P, Morgan-Joseph T. Etesevimab. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2021.
- Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus Etesevimab in mild or moderate covid-19. New England Journal of Medicine 2021. DOI: 10.1056/NEJMoa2102685

