Epidemiological Study of Intestinal Parasitism in the Child Population of Department of Managua (Nicaragua)
Mónica Gozalbo*, Aleyda Pavón, Rafael Toledo, Carla Muñoz-Antolí, José Guillermo Esteban
Department of Preventive Medicine and Public Health, Science of Food, Toxicology and Forensic Medicine, University of Valencia, Spain
Department of Clinical Bioanalysis, Instituto Politécnico de la Salud (IPS-Polisal), Autonomous University of Nicaragua, Managua, Nicaragua
Aarea of Parasitology, Department of Pharmacy and Technology Pharmacology and Parasitology, University of Valencia, Spain
Received Date: 02/10/2021; Published Date: 05/11/2021
*Corresponding author: Mónica María Gozalbo Monfort, Parasitology. Area of Nutrition and Chromatology, Department of Preventive Medicine and Public Health, Science of Food, Toxicology and Forensic Medicine, University of Valencia, 46100 Burjassot, Spain
Abstract
This study is part of a macroproject that aims to know the coproparasitological status and its correlation with epidemiological data and behavioral habits of the Nicaraguan child population. This contribution studies a total of 1,936 subjects (1,022 boys and 914 girls) aged between 1 and 15 years from the Department of Managua (Nicaragua), from different schools and neighborhoods, differentiating the population under study according to their origin (urban areas central and peripheral and rural areas). The coproparasitological study, performed on a fecal sample per subject, has made it possible to detect a parasitic spectrum consisting by 20 different parasitic species (11 protozoa and 9 helminths), with a total parasitation prevalence of 71.0%, by protozoa of 69.7% and by helminths of 9.2%. The most prevalent species were Blastocystis hominis (48.6%), Entamoeba coli (29.0%), and Giardia intestinalis (25.1%). Has been found a clear predominance of multiparsitism (65.7%) about monoparasitism (34.3%), with a case of parasitation by 10 different species. The influence of age, sex and different socioeconomic and hygienic-health factors that can pose a risk factor for the acquisition of enteroparasitosis has been analysed. The results obtained have been contrasted not only with the limited literature that has so far in the Nicaraguan children's population, but also in the Central American and Caribbean areas. The study carried out allows to highlight how positives it is, from the parasitological side, the children's campaigns of helminthic deworming carried out by the Government of Nicaragua, at least in the Department of Managua, taking advantage of child vaccination campaigns, although environmental sustainability policies must be addressed to improve the health of nicaraguan children.
Keywords: Enteroparasites; Protozoa; Helminths; Prevalence; Epidemiology; Risk factors; Department of Managua; Nicaragua
Introduction
WHO reports show that among infectious diseases affecting humanity, intestinal parasitosis is a major global health problem (WHO, 2010, 2017) [1]. Its importance lies in its high prevalence due to the intestinal microhabitat where the parasites are located and are able to cause serious gastrointestinal damage as well as complications such as severe anemic symptoms, stunting, affliction of cognitive functions, even compromising productivity (Ahmed et al., 2016; Zavala et al., 2016) [2,3]. In addition, they have a mechanism of direct transmission either through anus-hand-mouth contamination, through the skin or through contamination and mishandling of food or water, which has a profound impact on a large section of populations of low economic, educational and social conditions. Basic services do not exist or are in a lamentable state; and the impact is felt significantly more in children than in adults, which relates to poor or inadequate hygiene and behavioral habits (Pawar et al, 2016; Speich et al., 2016; Pabalan et al., 2018) [4-6].
Enteroparasitoses are sensitive and objective indicators of ecological factors, the degree of environmental sanitation and the cultural, economic and social conditions of individuals. Their higher prevalence in tropical regions and poorer communities has led to the tendency to consider these infections only as a product of living conditions, with their impact being underestimated by public health services (Barretto et al., 2012; Kenyin & Santiago, 2013) [7,8]. It should be noted that mortality is low, and it is estimated that about one billion people worldwide are infected with at least one enteroparasite species (WHO, 2010) [9].
The interest of the study of enteroparasitosis in department of Managua (Nicaragua) is evidenced by its manifest importance in public health, coupled with the scarcity of studies on intestinal parasitism in Central America in general, and Nicaragua in particular. Although the country's economic progress has reduced the magnitude and severity of its poverty, Nicaragua continues to be the second poorest and underdeveloped country in the Americas (Banco Mundial, 2020) [10].
A thorough review of studies carried out in that country shows the scarcity of work on the subject. Data accessible to the scientific community in specialized journals are minimal. There are studies limited to the departments of Carazo, León, Managua and Rio San Juan in the Pacific Region, and Corn Island and Laguna de Perlas located in the Atlantic Region (Duarte et al., 1991; Cavouti & Lancaster, 1992; Téllez et al., 1992, 1997; Oberhelman et al., 1998; Leiva et al., 2008; Lebbad et al., 2008; Rosewell et al., 2010; Muñoz-Antoí et al., 2011; Karan et al., 2012; Muñoz-Antoli et al., 2014, 2017, 2018a, 2018b) [11-24], with the studies of Muñoz-Antolí et al. (Muñoz-Antoli et al., 2011, 2014, 2017, 2018a, 2018b) [19,21-24] standing out as part of this macro-project of cooperation, which aims to shed light on the intestinal parasites detected in the country’s child population, in order to determine the epidemiology of these infections and contribute to the diagnosis, treatment and control..
The aim of this study, focused on Department of Managua, is to discern the intestinal parasite spectrum in the child population and to expose the risk factors associated with these parasitoses.
Material and Methods
Study area and population
This study was conducted between 2005 and 2007 in Department of Managua (12º08'00'O 86º15'00'W), with Managua as capital of the department, and covering an area of about 3,495 km2,, located on the coast of the Pacific Ocean. The area borders the Departments of Matagalpa and León to the north, the Pacific Ocean and Department of Carazo to the south, the Departments of Boaco, Granada and Masaya to the east, and the Department of León to the west. The total population is 1,262,978 inhabitants, with an overwhelming urban population of 90.5%, and a small rural population of 9.5% (INEC, 2005) [25]. Only 32.1% of the population in the department is below 15 years of age, as opposed to a large percentage of an active population of 63.6% in the range of 15 to 64 years of age, and being lower than the population of elderly citizens aged 65 and above, 4.3%. The local economy is based on agriculture and industry mainly located around the municipalities of Managua and Tipitapa. The climate is typical humid tropical with high temperatures and abundant rainfall, with a very short dry season (March to June).
The surveys were conducted in five municipalities out of the nine that make up the department. Urban areas were distinguished from rural ones considering the following criteria: usually having paved roads, street lighting, well or tap water, latrines, a sewer system or wastewater that runs through the streets, homes made of cement, and having medical services at their disposal. In contrast, rural areas usually have dirt roads, well or river water, latrines are rare and defecation takes place outdoors, wastewater runs through the streets and courtyards, and homes have dirt floors. However, within urban areas, downtown areas are characterized by neighborhoods with electricity supply and public lighting, drinking water and their streets are in good conditions, providing relatively healthy living conditions, while peripheral areas have drinking water and street lighting, and settlements appear spontaneously with the accumulation of unauthorized garbage.
The coprological survey involved 1,936 children (1,022 boys and 914 girls) from 1 to 15 years of age, coming from the different municipalities. Managua municipality (12º09'00'O 86º16'00'W) covering 267,17 km2 and with 937,489 inhabitants, with a total of 1,058 children analyzed: 866 coming from the urban area (482 in downtown areas and 384 in peripheral area) and 192 of rural area origin. The remainder of the municipalities were all of the rural area: Villa El Carmen (11º59'00'O 86º31'00'W) covering 562,01 km2 and having 27,449 inhabitants, with a rural population of 88.0%, of whom 200 children were studied; Ticuantepe (12º01'00'O 86º12'00'W) covering 68 km2 and having a total population of 27,008 inhabitants, with 61.2% of rural areas where 193 children were studied; El Crucero (11º59'00'O 86º19'00'W) covering 225,72 km2 and having 13,656 inhabitants, with a rural population of 21.1%, where 159 children were studied; and finally, the municipality of Tipitapa (12º12'00'O 86º06'00'W) covering 975,30 km2 and having 101,685 inhabitants, with a rural population of 50%, where a total of 326 children wre studied.
Data collection and laboratory methods
The teachers and students at each school were informed about the objectives of the study. In the case of neighborhood collections, the basics of the study were explained to the members of each home included. The total samples collected were representative of the number of students enrolled at each school and the child population of each ward, which would allow adapting the strategic design of a study to the child population of different environments (urban and rural) and different socio-economic conditions, always within Department of Managua.
Each student and neighborhood children were given a clean plastic container, wide-mouthed, numbered, and with a pressure cap. They were then asked to fill the container with their own feces and return it the next day. A single stool sample was obtained from each child, and questionnaires, concerning personal data (sex and age), epidemiological data on health (water supply: well or river water; and sanitation: latrine or outdoor defecation) and behavioral habits (eating with unwashed hands, eating raw fruits and vegetables without washing and walking barefoot), were distributed among the participants.
Each stool sample was fixed in situ with a 10% formalin solution and posteriorly filtered one by one through gauze. The sediment was then deposited in plastic bottles, with screw cap, and properly labeled. Finally, all the samples were sent to Department of Parasitology of University of Valencia (Valencia, Spain) for processing and analysis (Ash et al., 1994) [26]. Each fecal sample was concentrated with the modified formalin-ether method (Knight et al., 1976) [27] and the sediment was analyzed, i.e., at least 2 drops of each fecal samples. A fecal smear of the sediment was carried out, being stained with the modified Ziehl-Neelsen technique (Ash et al., 1994) [26].
Statistical analysis
Statistical analysis was carried out using the Epi Info version 6.1 program (CDC, Atlanta, USA). The statistical comparison of categorical or qualitative variables was performed with the c2 test. Associations between protozoa and Soil-Transmitted Helminth (STH) infections were determined by contingency tables 2×2. In addition, the Odds Ratio (OR) with a 95% confidence interval was evaluated by univariate analysis to determine the significance levels of all considered risk factors and their impact on the intestinal parasitism. A p-value of 0.05 was considered statistically significant.
Ethical considerations
Informed consent was obtained from the parents, respectively, guardians of the enrolled schoolchildren of each ward. The results of the diagnosis were sent to Instituto Politécnico de la Salud, which informed the Nicaraguan Ministry of Health about appropriate treatments.
Results
Parasite study
Table 1 summarizes the results obtained by urban and rural areas and in the total study. The entire spectrum consisted of 20 species of parasites. It should be noted that in 71.0% of the children surveyed, an infection with at least one parasite species, whether protozoa (69.7%) or helminths (9.2%), was found.
Table 1: Prevalence of parasitization in the total population studied in Department of Managua (N=number of total individuals studied; n=number of parasitized individuals; %=percentage of parasitization; I.C. 95%==95% confidence interval).
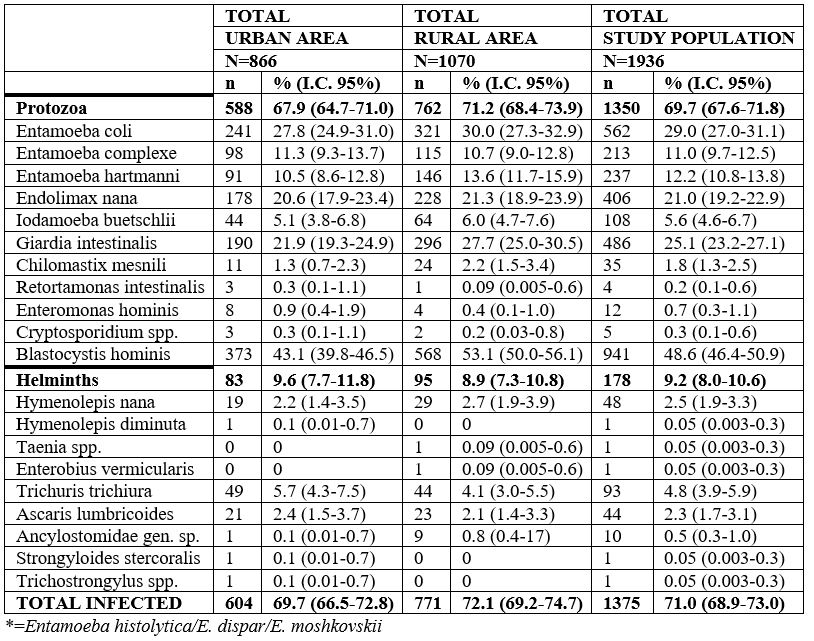
Blastocystis hominis (48.6%) was the most commonly diagnosed intestinal parasite species, followed by Entamoeba coli (29.0%) and Giardia intestinalis (25.1%). Among helminths, Trichuris trichiura, Hymenolepis nana and Ascaris lumbricoides, were the most prevalent (4.8%, 2.5% and 2.3%, respectively). Particular attention should be paid to the prevalence results of Enterobius vermicularis and Strongyloides stercoralis, which cannot be considered definitive as adequate techniques were not used for the detection of these nematode species.
No statistical differences were observed in the comparative analysis of prevalence rates detected in both areas (urban and rural). However, a higher prevalence was detected in the rural area than in the urban area, as well as statistical differences in certain protozoa, i.e. G. intestinalis (c2=8.34; p=0.03879) and B. hominis (c2=19.21; p=0.000012). Concerning helminths, no statistical differences were found between the two areas.
Data obtained from locations in the urban area are gathered in Table 2. In downtown area, the parasite spectrum was composed of 16 species. 62.4% of children presented parasitation, 59.8% by protozoa and 9.5% by helminths. Among protozoa, the most prevalent were B. hominis (30.9%), E. coli (25.9%) and G. intestinalis (19.7%), while among helminths, the most prevalent were T. trichiura (5.4%), A. lumbricoides (2.7%) and H. nana (1.9%). In the peripheral area, a parasite spectrum of 14 species was detected, with a global parasitation of 79.0%: 78.1% affected by protozoa and 10.2% by helminths. Among protozoa, B. hominis (58.3%), E. coli (30.2%), E. nana (28.9%) and G. intestinalis (24.7%) are the ones that presented the highest prevalences. And among helminths, T. trichiura (5.9%), H. nana (2.6%) and A. lumbricoides (2.1%) were the most frequent.
Table 2: Prevalence of parasitization in the urban area, according to downtown o peripheral area, respectively, in the municipality of Managua (N=Number of total individuals studied; n=number of parasitized individuals; %=percentage of parasitization; I.C. 95%=confidence interval 95%).

Comparative analysis of the prevalence rates detected in the urban area showed higher percentages in the peripheral area than in the downtown, with statistical differences in the total infection of parasitic speciess (c2=27.44; p<0.000001), and in protozoa infection (c2=33.10; p<0.000001), specifically by Entamoeba complex (c2=21.64; p=0.000003) and B. hominis (c2=5.54; p<0.000001). No statistical differences were found in helminth infections.
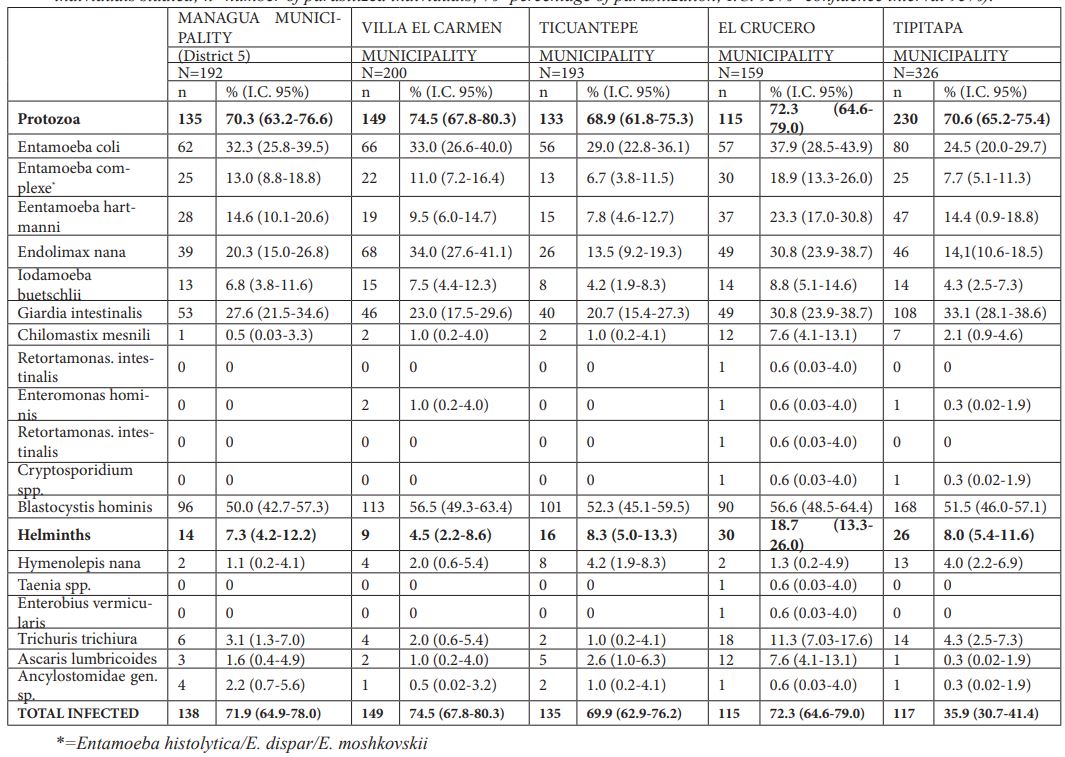
In the rural area (see Table 3), the parasite spectrum consisted of 17 species, with 72.1% of the children suffering infection, i.e., 71.2% protozoa and 8.9% helminth infections. The most prevalent protozoa were, similar to the urban area, B. hominis (53.1%), E. coli (30.0%) and G. intestinalis (27.7%). For helminths, the highest prevalence was observed in T. trichiura (4.1%), followed by H. nana (2.7%), A. lumbricoides (2.1%) and Ancylostomatidae gen. sp. (0.8%).
Table 3: Prevalence of parasitization in the five municipalities of the rural area of Department of Managua (N=Number of total individuals studied; n=number of parasitized individuals; %=percentage of parasitization; I.C. 95%=confidence interval 95%).
Referencing the total number of parasites found, the highest prevalence of parasitism was observed with a single parasite species (34.3% [472/1375]) with a statistically significant difference when compared to parasitism by two species (30.1% [414/1375]) (c2=579; p=0.01609). It should be noted that multiparasitism by 10 associated parasite species was detected in a 10-year-old boy from the rural area and the highest number of associated protozoa were those of a 12-year-old girl from the peripheral urban area who habored 8 species of protozoa simultaneously. In the study of the total number of helminth species found in the same individual, monoparasitism (11.4% [157/1375]), parasitism by 2 species (1.5% [21/1375]) and by 3 species (0.1% [1/1375]) were detected, with statistically significant differences between them, with monoparasitisms presenting the highest prevalence (c2=252.48; p<0.05). The highest number of helminth species was observed in a 5-year-old boy from the peripheral urban zone harboring a total of 3 helminths species: T. trichiura, H. nana and S. stercoralis.
Epidemiological variables
With regard to sex (see Table 4), no statistical significance was detected in any of the particular items (total parasitation, total parasitation by protozoa and total parasitation by helminths), although a higher prevalence of parasitation by certain protozoa was observed in females with a statistical significance, although they were almost always commensal protozoa. The Odds Ratio with respect to sex determined that neither being female [1.01 (0.83-1.24)] nor male [0.99 (0.81-1.21)] could be considered a risk factor favoring parasitation in any of the locations studied. Thus, it can be asserted that both sexes are equally susceptible to being parasitized in Department of Managua.
Table 4: Prevalence of parasitation with respects to sex of the population studied in Department of Managua (N=Number of total individuals studied; n=number of parasitized individuals; %=percentage of parasitation; I.C. 95%=confidence interval of 95%).

The parasite spectrum with respect to age group distribution was analyzed (Table 5). Schoolchildren (6-11 years) and adolescents (>11 years) were more parasitized, and statistical significances with respect to infants (0-5 years) were detected. Specifically, the statistical differences appeared in relation to the parasitized total (c2=57.11; p<0.000001); total parasitation by protozoa (c2=52.56; p<0.000001), in particular with E. coli (c2=35.78; p<0.000001), Entamoeba complex (c2=25.32; p=0.000003), and B. hominis (c2=45.53; p<0.000001); and the total parasitation by helminths (c2=14.36; p=0.000761). Only in the case of G. intestinalis (c2=9.94; p=0.006926), was the highest prevalence of parasitation obtained in infants (0-5 years).
When calculating the Odds Ratio among the age groups of the total child population studied, both age ranges, schoolchildren (6-11 years) and adolescents (>11 years), can be considered risk factors in relation to intestinal parasitation ([1.57 (1.28-1.93)] and [1.47 (1.09-1.97)], respectively).
Table 5. Prevalence of parasitation with respect to age of the population studied in Department of Managua (N=Number of total individuals studied; n=number of parasitized individuals; %=percentage of parasitation; I.C. 95%=confidence interval of 95%).

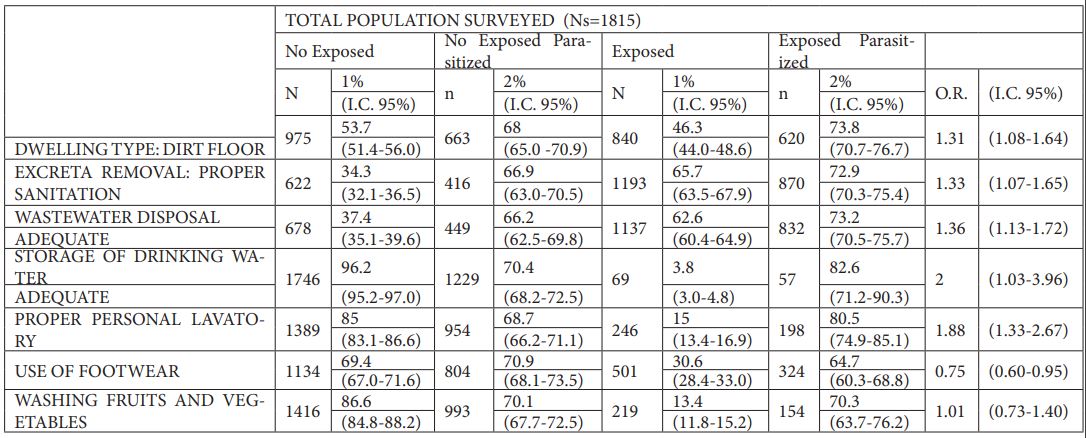
The analysis of socioeconomic conditions such as the type of floor of the dwelling and the sanitation system (excreta removal and wastewater drainage) allowed obtaining the Odds Ratio for the total population studied. Inhabiting a dwelling with a dirt floor [1.13 (1.08-1.64)], inhabiting a dwelling with inadequate sanitation or inadequate excreta removal [1.33 (1.08-1.66)], inadequate wastewater disposal [1.36 (1.10-1.69)] and inadequate storage of drinking water [2.10 (1.06-4.29)] should be considered risk factors for intestinal parasitation in Department of Managua (see Table 6).
Personal hygiene habits (personal grooming and footwear use) and food hygiene habits (washing fruits and vegetables) were analyzed to assess whether non-compliance with minimum hygiene measures should be considered a risk factor for school-age children in relation to intestinal parasites. It was found that improper personal hygiene [1.88 (1.33-2.67)] should be considered a risk factor, while not wearing shoes [0.75 (0.60-0.95)] and not washing fruits and vegetables before consumption [1.16 (0.83-1.63)] should not be considered risk factors for contracting enteroparasites in Department of Managua (Table 6).
Table 6. Odds Ratios related to extrinsic factors of the population studied (Ns=number of individuals surveyed; N=number of individuals in the given group; n=number of individuals in the given group parasitized; %1=percentage of parasitation between N and Ns; %2=percentage of parasitation between n and N; I.C. 95%=95% confidence interval; O.R.=Odds Ratio value).
Discussion
In the context of the country and its manifest poverty, the total prevalence of parasitation in the child population of Department of Managua (71.0%) was very high, with a parasite spectrum consisting of 20 parasite species (11 protozoa and 9 helminths but without trematodes) and a noticeable percentage of multiparasitism to a different degree, of up to 10 different parasite species. Among the most medically relevant parasite species are those reaching the highest prevalences, B. hominis (48.6%) and G. intestinalis (25.1%); and for helminths, the highest prevalences were observed in T. trichiura (4.8%), H. nana (2.5%) and A. lumbricoides (2.3%). In view of these data, the study carried out in Department of Managua yields figures that are above those detected in other departments of equal characteristics of development and population, such as the Departments of León and Carazo (Téllez et al., 1997; Oberhelman et al., 1998; Rosewell et al., 2005) [14,15,18] and actually approaching values closer to those known in other departments of the Pacific Region: Rio San Juan, Corn Island or Laguna de Perlas (both located in the South Atlantic Autonomous Region, RAAS), where socio-economic levels and hygienic-sanitary conditions are extremely low and deficient (Téllez et al., 1992; Muñoz-Antolí et al., 2014, 2017, 2018a, 2018b) [13,21-24]. Compared, in particular, to the results obtained with those in other studies conducted in different locations in Nicaragua, Entamoeba complejo showed the lowest prevalence of parasitation (0.1%) in the Department of Carazo (Oberhelman et al., 1998) [15], while in Department of León it was G. intestinalis (15.6%) (Téllez et al., 1997) [14]. The highest prevalence of parasitation was determined in Río San Juan and Laguna de Perlas for B. hominis (77.7% and 58.1%, respectively) and G. intestinalis (32.5% and 17.4%, respectively) (Muñoz-Antolí et al., 2014; 2017) [21,22]. The study of Muñoz-Antolí et al. (2018a) [23] carried out in 7 departments in Nicaragua's Pacific Region, in children until 5-year age group, B. hominis and G. intestinalis also stood out with prevalence values between 32-70% and 21-28%, respectively. The prevalence of T. trichiura was higher than that of A. lumbricoides, a fact that coincides with other works carried out in Nicaragua (Cavouti & Lancaster, 1992; Rosewell et al., 2010; Muñoz-Antolí et al., 2014, 2017, 2018a, 2018b)[12,18,21-24], whilst being in contrast to the results obtained by Oberhelman et al. (1998) [15] in Department of Carazo. When considering geohelminths only, the low prevalences of parasitation obtained for ancylostomatides (0.5%) and S. stercoralis (0.05%), especially with regard to other studies carried out in other departments (between 0.1 % and 18.6%) (Téllez et al., 1992; Rosewell et al., 2010; Muñoz-Antolí et al., 2014, 2017, 2018b) [13,18,21,22,24], is noteworthy. It should be noted that a broad spectrum of parasite species without medical relevance was determined, i.e., E. coli, E. hartmanni, E. nana, I. buestchlii, Ch. mesnili, R. intestinalis and E. hominis, but which have epidemiological connotations as a result of oral-fecal transmission, or person-to person, which clearly implies poor hygienic-sanitary conditions in the population studied in both, the urban and rural areas of Department of Managua. This presence of clinically irrelevant parasitic species was also encountered in the studies of Muñoz-Antolí et al. (2014, 2017, 2018a) [21-23] carried out hitherto in urban as well as rural areas of Nicaragua. Similar results were reached in a study conducted in Guatemala where higher parasitation prevalences were observed in densely populated locations
When comparing the results obtained to the Central America and Caribbean island studies (Reyes et al., 1987; Reinthaler et al., 1988; Kaminsky, 1991; Holland et al., 1998; Kaminsky et al., 1998, 2014; Cruz et al., 1998; Morales-Espinoza et al., 2003; Núñez et al., 2003a; Sánchez-Vega et al., 2006; Lavin et al., 2008; Cook et al., 2009; Jensen et al., 2009; Cañete et al., 2012; Quihui & Morales, 2012; Sandoval et al., 2015; Perea et al., 2020) [28-43] with a global spectrum consisting of 16 parasite species, i.e., 10 protozoa and 8 helminths, greater qualitative wealth was found in this study. These results are even more noticeable when considering the fact that only one coprological sample was analyzed per child, which underestimates the actual prevalence of intestinal parasite species, as protozoan cysts/oocysts and helminth eggs and larvae are expelled intermittently. It should be noted that B. hominis did not turn out to be as common in most studies of these locations, possibly due to the inability or difficulty of recognizing and microscopically identify this species, resulting in prevalences between 1.9 and 38.9% (Reinthaler et al., 1988; Cruz et al., 1998; Kaminsky et al., 1998; Núñez et al., 2003a; Lavin et al., 2008; Cook et al., 2009; Cañete et al., 2012; Sandoval et al., 2015; Perea 2020) [29,32,33,35,37,38,40,42,43]. In contrast, the prevalence of G. intestinalis was lower compared to most Central America and Caribbean island studies showing a parasitation of between 29.0% and 61.0% (Reyes et al., 1987; Kaminsky, 1991; Sánchez-Vega et al., 2006; Cañete at al., 2012; Quihui & Morales, 2012) [28,30,36,40,41]. For Entamoeba complejo, mixed results (between 4.5% and 79.7%) (Morales-Espinoza et al., 2003; Sánchez-Vega et al., 2006; Quihui & Morales, 2012; Perea et al., 2020) [34,36,41,43] were found, while T. trichiura and A. lumbricoides were among the most determined geohelminths (Reinthaler et al., 1988; Holland et al., 1998; Jensen et al., 2009; Kaminsky et al., 2014) [29,31,39,44] albeit with lower prevalences than protozoa, due to anthelmintic campaigns carried out in all Latin American countries (Quihui & Morales, 2012) [41].
When considering the sex variable, a higher prevalence of parasitation was detected in the female sex than in the male sex, although only in relation to certain protozoa, likely to be due to occupational and behavioral factors and not a susceptibility inherent to sex, since an Odds Ratio below 1 was obtained both with respect to the male as well as female sex. These results contrast with those provided by some authors in different countries of the Central America and Caribbean island where belonging to a certain sex is not a risk factor for parasitic infection in general, respectively, by protozoas or by helminths, in particular (Cook et al., 2009; Lavin et al., 2008; Quihui & Morales, 2012; Jensen et al., 2009; Kaminsky et al., 2014; Champetier de Ribes et al., 2005; Quihui et al., 2016) [37-39,41,44-46].
The highest overall prevalences of parasitation, in both protozoa and helminths, occur in children above 5 years of age in all geographical areas studied. both urban and rural, although statistically significant differences were not detected between helminth species and the different age groups considered. These results coincide with previous works in the population of Central America and Caribbean islands (Nuñez et al., 2003a, 2003b; Kaminsky et al., 2014) [35,44]. The highest prevalences in the age group below 5 years of age were those of G. intestinalis and Cryptosporidium spp., although without statistically significant differences, being similar to other studies (Cifuente et al., 2000; Corrales et al., 2006; Escobedo et al., 2008; Cook et al., 2009; Cañete et al., 2012) [38,40,47-49]. Although it should be noted that the age variable was a risk factor with regard to intestinal parasitosis in the infant population over 5 years, which is usually about the age when children begin to socialize with other individuals, away from the continuous care of parents.
The socioeconomic conditions surrounding individuals and the hygienic-sanitary conditions inherent to an individual were studied. Socioeconomic factors play an important role in the transmission of enteroparasitosis as they facilitate the evolution, spreading and maintenance of the different evolutionary forms that are part of the biological cycle of each parasite species (Kaminsky et al., 1998; Asaolu & Ofoezie, 2003; Jarquin et al., 2016; Galván-Ramírez et al., 2019; Zavala et al., 2020) [32,50-53].
In Department of Managua, in general, living a dwelling with dirt floor was a risk factor for contracting intestinal parasitosis. Similar circumstances were documented in various Central America and Caribbean studies (Forrester et al., 1990; Anderson et al., 1993; Holland et al., 1998; Corrales et al., 2006; Galván-Ramírez et al., 2019) [31,48,52,54,55]. This type of floor is an ideal place to keep eggs and cysts viable, which can be transported over long distances on a person’s feet, hands or shoes and deposited inside or around the dwelling.
The relationship between inadequate excreta disposition systems and the acquisition of intestinal parasitosis was a risk factor in Department of Managua. Several studies coincide with these results (Reyes et al., 1987; Forrester et al., 1990; Kaminsky, 1991; Anderson et al., 1993; Cruz et al., 1998; Kaminsky et al., 1998; Cifuentes et al., 2000; Núñez et al., 2003b; Corrales et al., 2006; Quihui et al., 2016; Galván-Ramírez et al., 2019; Sorto et al., 2015) [28,30,32,33,46-48,52,54-57]. If expelled pathogens are not eliminated by adequate sanitation, they can survive in soil, ponds, etc., remaining infective. It should also be noted that the use of human or animal feces as fertilizer is very common, being a potential risk of infection (Silva et al., 2008) [58].
Another determining factor in the transmission of parasite diseases is the consumption of water (Cañete et al., 2012; Damiani et al., 2013) [40,59], which is the cause of the occurrence of epidemic episodes as well as of the maintenance of parasites, along with other factors of situations of endemicity (Núñez et al., 2003b) [56]. Especially protozoa that play a greater role in water transmission by having resistant forms. Most enteric diseases detected in Central America and the Caribbean islands are related to inadequate water supply and storage (Reyes et al., 1987; Aimpun & Hshieh, 2004; Cifuentes et al., 2004; Zavala et al., 2020) [28,47,53,60]. The results of this study found that consuming "poor quality" water (preserved uncovered) was a risk factor for the acquisition of enteroparasitoses. The reuse of wastewater for irrigation, a common practice in arid countries, which can cause infecting forms of parasites to be viable in some produce grown at ground level, which may pose a risk of infection for human populations (Cifuentes et al., 2000; Blumenthal et al., 2001) [47,61].
Poor hygienic-sanitary conditions also allow the proliferation of parasitic diseases. Personal hygiene encompasses hand washing before eating, after defecation, and having a daily bath or shower. These simple hygiene practices significantly reduce the risk of enteroparasitoses. Inadequate personal hygiene poses a risk factor in the overall population studied, indicating that the minimum hygienic conditions that could decrease or eliminate enteroparasitoses are neither followed at home nor at school. In addition, another well-proven fact is that children of these ages, regardless of their sex, become sociable in exchanging food and beverages, further promoting transmission. Some studies in Central America and the Caribbean coincide with the findings obtained in this work (Cruz et al., 1998; Cifuentes et al., 2004; Cañete et al., 2012, WHO., 2005) [33,40,62,63].
The consumption of raw unwashed fruits and vegetables, which is related to the frequent practice of using, for irrigation in agriculture, water contaminated with faecal material or even the use of faecal matter as fertiliser of fields, is highlighted (Cañete et al., 2012; Jarquin et al., 2016) [40,51]. Epidemiological data obtained in Department of Managua do not show as a risk factor the incorrect washing of fruits and vegetables before consumption.
Conclusion
Of the three pillars on which the prevention, fight and control of intestinal parasitoses is based, it is considered essential to enhance and invest in environmental sustainability, i.e. the provision of acceptable drinking water and the provision of excreta disposal systems or to proceed, if not, to the construction and use of latrines. This measures of environmental sustainability must be focused on the entirety of Department of Managua. The basic role of timely and relevant education and information for both urban and rural communities should be strengthened, particularly with regard to pollution of the teluric and water environment and the application of adequate personal hygiene measures, especially in the female sex and in the population over 5 years of age. And finally, the marked differences that the qualitative and especially the quantitative level, detected between the two parasitic groups protozoa and helminths, show is the positive role the Nicaraguan government plays with the helmintic deworming program in children carried out along with the childhood vaccination campaigns in most of the country’s departments.
Conflictis of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgement
We are very grateful to the principals and teachers of each school, and the parents of the schoolchildren who have participated in this study.
The funds for this study were obtained from the Development Cooperation Project 2007 (s/n) of the Vice-Chancellor of Institutional Relations and Cooperation of University of Valencia-Estudi General (Valencia, Spain).
Declaration of Interests
None
References
- World Health Organization. Integrating neglected tropical disease into global health and developement fourth WHO report on neglected tropical disease, World Health Organitzation, Geneve. 2017; pp.267.
- Ahmed T, Khanum H, Uddin MS, Barua P, Arju T, Kabir M, Entamoeba histolytica, Giardia lamblia and Cryptosporidium spp., infection in children in an urban slum area of Bangladesh. BRC, 2016; 2: 175-181.
- Zavala GA, Rosado JL, Colleen MD, Caamaño MC, Campos-Ponce M, Ronquillo D, et al. Energy and food intake are associated with specific intestinal parasitic infection in children of rural Mexico. Parasitol Int, 2016; 66: 831-836.
- Pawar S, Ingole K, Bhise M. Study of prevalence of intestinal parasitic infection in symptomatic children at tertiary care hospital. Int J Appl Res, 2016; 2: 243-248.
- Speich B, Croll D, Furst T, Utzinger J, Keises J. Effect of sanitation and wáter treatment on intestinal protozoa infection a systematic reviiew and meta-analysis. Lancet Infect. Dis, 2016; 16: 87-99.
- Pabalan N, Singian E, Tabangay L, Jarjanazzi H, Boivin MJ, Ezeamama AE. Soil-transmitted helminth infection loss of education and cognitive impairment in school-aged children: A systematic review and meta-analysis. PLoS Negl Trop Dis, 2018; 12: e0005523.
- Barretto SM, Miranda JJ, Figueroa JP, Schimdt MI, Muñoz S, Kuri-Mordes PP, et al. Epidemiology in Latin American and the Caribean. Current situation and challenges. Int J Epidemiol, 2012; 41(2): 557-571.
- Kenyin S, Santiago E. El abordaje integral de las enfermedades tropicales desatendidas en América Latina y el Caribe. Un imperativo ético par alcanzar la justicia y la equidad social. Biomédica, 2013; 30: 159-163.
- World Health Organization. Working to overcome the global impact of neglected tropical diseases: First WHO report on neglected tropical diseases, World Health Organization, Geneva, 2010; pp.140.
- Banco Mundial, Nicaragua: panorama general, 2020.
- Duarte Z, Morera P, Voung PN. Abdominal angiostrongyliasis in Nicaragua: a clinico-pathological study on a series of 12 cases reports. Ann Parasitol Hum Comp, 1991; 66: 259-262.
- Cavouti D, Lancaster KR. Intestinal parasitism of children on Corn Island, Nicaragua. Pediat Infect Dis. J, 1992; 11: 775-776.
- Téllez A, Cortes L, Aust A, Huldt G, Honsson J, Schroder H. Amebiasis in Nicaragua: class specific serum antibody responses. Arch Med Res, 1992; 23: 261-264.
- Téllez A, Morales W, Rivera T, Meyer E, Leiva B, Linder E. Prevalence of intestinal parasites in the human population of Leon, Nicaragua. Acta Trop, 1997; 66: 119-125.
- Oberhelman RA, Guerrero ES, Fernández ML, Silio M, Mercado D, Comiskev N, et al. Correlations between intestinal parasitosis, physical growth, and psicomotor development among infants and children from rural Nicaragua. Am J Trop Med Hyg, 1998; 58: 470-475.
- Leiva B, Lebbad M, Winiecka-Krusnell J, Altamirano I, Téllez A, Linder E. Overdiagnosis of Entamoeba histolytica and Entamoeba dispar in Nicaragua: A Microcopic, Triade Parasite Panel and PCR Study. Arch Med Res, 2006; 37: 529-534.
- Lebbad M, Ankarklev J, Téllez A, Leiva B, Anderson JO, Avärd S. Dominance of Giardia assemblage B in León, Nicaragua. Acta Trop, 2008; 106: 44-53.
- Rosewell A, Robleto G, Rodríguez G, Barragne-Bigot P, Amador JJ, Aldighieri S. Soil-transmitted helminth infection and urbanization in 880 primary school children in Nicaragua, 2005. Trop Doctor, 2010; 40(3): 141-143.
- Muñoz-Antolí C, Pavón A, Marcilla A, Toledo R, Esteban JG. Prevalence and molecular characterization of Cryptosporidium in schoolchildren from departamento of Río San Juan. Trop Biomed, 2011; 28: 40-47.
- Karan A, Chapman GB, Galvani A. The influence of poverty and culture on the transmission of parasitic infections in rural nicaraguan village. J Parasitol, ID 478292, 2012; pp.12.
- Muñoz-Antolí C, Pavón A, Marcilla A, Toledo R, Esteban JG. Prevalence and risk factors related to intestinal parasites among children in Department of Rio San Juan, Nicaragua. Trans R Soc Trop Med Hyg, 2014; 108: 774-782.
- Muñoz-Antolí C, Pavón A, Pérez P, Toledo R, Esteban JG. Soil-transmitted Helminth Infections in Schoolchildren of Laguna de Perlas (Nicaragua). J Trop Pediat, 2017; 63: 124–134.
- Muñoz-Antolí C, Gozalbo M, Pavón A, Pérez P, Toledo R, Esteban JG. Enteroparasites in Preschool Children on the Pacific Region of Nicaragua. Am J Trop Med Hyg, 2018a; 98: 570-575.
- Muñoz-Antolí C, Pérez P, Pavón A, Toledo R, Esteban JG. Soil-transmitted helminths and anemia in Schoolchildren from Corn Island Archipelago (RAAS, Nicaragua). Am J Trop Med Hyg, 2018b; 99(6): 1591-1597.
- Instituto Nacional de Estadísticas y Censos (INEC), VIII Censo de Población y IV de vivienda. Caracterización sociodemográfica del departamento de Managua. Instituto Nacional de Información de Desarrollo. Gobierno de Nicaragua, 2005; 110pp.
- Ash L, Orihel TC, Savioli L. Bench Aids for the Diagnosis of Intestinal Parasites. Geneve. Switzerland: World Health Organitzation, Geneve, 1994.
- Knight WB, Hiatt RA, Cline BL, Ritchie LS. A modification of the formol-ether concentration technique for increased sensitivity in detecting Schistosoma mansoni eggs. Am J Trop Med Hyg, 1976; 55: 818-823.
- Reyes L, Marín R, Catarinella G, Vargas A, Valenciano EN, Albertazzi C, et al. Parasitosis intestinal en niños en guarderías de San José, Costa Rica. Rev Costarric Ciénc Méd, 1987; 8: 123-128.
- Reinthaler FF, Linck G, Klem G, Mascher F, Sixl W. Intestinal parasites in children with diarrea in El Salvador. Geogr Med, 1988; 18: 175-180.
- Kaminsky RG. Parasitism and diarrhea in children from two rural communities and marginal barrio in Honduras. Trans R Soc Trop Med Hyg, 1991; 85(1): 70-73.
- Holland CV, Taren DL, Crompton DET, Beisheim MC, Sanjur D, Barbeau I, et al. Intestinal helminthiases in relation to the socioeconomic environment of Panamanian children. Soc Sci Med, 1998; 26: 209-213.
- Kaminsky RG, Flores R, Alberto S, Milla V. Prevalencia de parasitismo intestinal en diferentes poblaciones de Honduras. Rev Méd Hondur, 1998; 66: 62-70.
- Cruz V, Morán C, Álvarez R. Parasitosis intestinal en niños de una comunidad rural y factores de riesgo implicados en ellas. Rev Mex de Pediatría, 1998; 65: 9-11.
- Morales-Espinoza EM, Sánchez-Pérez HJ, García-Gil MM, Vargas-Morales G, Méndez-Sánchez JD, Pérez-Ramírez M. Intestinal parasites in children, in highly deprived áreas in the border región of Chiapas, Mexico. Salud Pública Méx, 2003; 45: 379-388.
- Núñez FA, González MO, González I, Escobedo A, Cordoví RA. Intestinal coccidian in cuba pediatric patients with diarrhea. Mem Inst Oswaldo Cruz, Río de Janeiro. 2003a; 98: 539-542.
- Sánchez-Vega JT, Tay-Zavala J, Aguilar-Chiu A, Ruiz-Sánchez D, Malagón F, Rodríguez-Covarrubias JA, et al. Cryptosporidosis and other intestinal protozoan infections in children less then one year of age in Mexico City. Am J Trop Hyg, 2006; 75(6): 1095-1098.
- Lavin J, Pérez A, Finlay CM, Sarracent J. Parasitismo intestinal en una cohorte de escolares en 2 municipios de Ciudad de La Habana. Rev Cubana Med Trop, 2008; 60: 37-80.
- Cook DM, Chad R, Eggett DL, Booth GM. A retrospective analysis of prevalence of gastrointestinal parasites among school children in the Palajunoj Valley of Guatemala. J Health Popul Nutr, 2009; 27: 31-40.
- Jensen LA, Marlin JW, Dyck DD, Laubach HE. Prevalence of multi-gastrointestinal infection with helminth, protozoan and Campylobacter spp. in Guatemalan children. J Infect Dev Ctries, 2009; 3: 229-234.
- Cañete R, Morales M, Avalos R, Laúd PM, Ponce FM. Intestinal parasites in children from a day care centre in Matanzas City, Cuba. PlosOne, 2012; 7(12): e51394.
- Quihui L, Morales G. Persistence of intestinal parasitic infections during the national de-worming campaign in schoolchildren of northwestern Mexico: a cross-sectional study. Ann Gastroenterol, 2012; 25: 57-60.
- Sandoval NR, Ríos N, Mena A, Fernández R, Perea M, Manzano-Román R, et al. A survey of intestinal parasite humans in Panama. Acta Trop, 2015; 147: 54-63.
- Perea M, Vásquez V, Pineda V, Samudio F, Calzada JE, Saldaña A. Prevalence and subtype distribution of Blastocystis sp. infecting children from a rural comunity in Panama. Parasite Epidemiol Control, 2020; 9: e00139.
- Kaminsky RG, Ault SK, Castillo Ph, Serrano K, Troya G. High prevalence of soil-transmitted helminth in Southern Belize-highlighting opportunity for control interventions. Asian Pac J Trop Biomed, 2014; 4(5): 345-353.
- Champetier de Ribes G, Flines M, Désormeaux AM, Eyma E, Montagut P, Champagne C, et al. Helminthoses intestinales en milieu scolaire en Haïti en 2002. Bull Soc Pathol Exot, 2005; 98: 127-132.
- Quihui L, Valencia ME, Crompton DWT, Phillips S, Hagan P, Morales G, et al. Role of the employment status and education of mothers in the prevalence of intestinal parasitic infections in Mexican rural schoolchildren. BMC Public Health, 2016; 6: 225-232.
- Cifuentes E, Gómez M, Blumenthal U, Téllez-Rojo MM, Romeu I, Ruiz-Palacios G, et al. Risk factors for Giardia intestinalis infection in agricultural villages practicing wasterwater irrigation in Mexico. Am J Trop Med Hyg, 2000; 62: 388-392.
- Corrales IF, Izurieta R, Moe LC. Association between intestinal parasitic infectious and type of sanitation system in rural El Salvador. Trop Med Int Health, 2006; 12: 1821-1831.
- Escobedo AA, Cañete R, Núñez FA. Prevalence, risk factors and clinical features associated with intestinal parasitic infections in children from San Juan y Martínez, Pinar del Río, Cuba. West Indian Med J, 2008; 57: 377-382.
- Asaolu SO, Ofoezie IE. The role of health education and sanitation in the control of helminth infections. Acta Trop, 2003; 86: 283-294.
- Jarquin C, Arnold BF, Muñoz F, López B, Cuéllar VM, Thornton A, et al. Population density, poor sanitation and enteric infection in Nueva Santa Rosa, Guatemala. Am J Trop Hyg, 2016; 94(4): 912-919.
- Galván-Ramírez ML, Madriz-Elisondo AM, Temores CG, Romero JJ, Carrasco DA, Cardona MA. Enteroparasitism and risk factors associated with clinical manifestations in children and adults of Jalisco State in Western Mexico. Osong Public Health Res Perspect, 2019; 10(1): 39-48.
- Zavala GA, van Dulm E, Doak CM, Garcia OP, Polman K, Campos-Ponce M. Ascariasis, amebiasis and giardiasis in Mexican children: distribution and geographical, environmental and socioeconomic. J Parasit Dis, 2020; 44(4): 829-836.
- Forrester JE, Scott ME, Bundy DAP, Golden MHN. Predisposition of individuals and families in Mexico to heavy infection with Ascaris lumbricoides and Trichuris trichiura. Trans R Soc Trop Med Hyg, 1990; 84: 272-276.
- Anderson TJC, Zizza CA, Leche GM, Scott ME, Solomons NW. The distribution of intestinal helminth infections in a rural village in Guatemala. Mem Inst Oswaldo Cruz, Río de Janeiro, 1993; 88: 53-65.
- Núñez FA, López JL, De la Cruz AM, Finlay CM. Factores de riesgo de la infección por Giardia intestinalis en niños de guarderías infantiles de Ciudad de La Havana, Cuba. Cad Saúde Pública, 2003b; 19: 677-682.
- Sorto OR, Portillo AM, Aragón AA, Saboya MI, Ade MP, Minero MA, et al. Prevalencia e intensidad de la infección por geohelmintos y prevalencia de la malaria en escolares de El Salvador. Biomédica, 2015; 35(5): 407-418.
- Silva J, Torres P, Madera C. Reuso de aguas residuales domésticas en agricultura. Una revisión. Agron Colomb, 2008; 26(2): 347-359.
- Damiani C, Balthazard-Accou K, Clervil E, Diallo A, Da Costa C, Emmanuel E, et al. Cryptosporidiosis in Haiti: surprisingly low level of species diversity revealed by molecular characterization of Cryptosporidium oocysts from surface water and groundwater. Parasite, 2013; 20(45): pp. 6.
- Aimpun P, Hshieh P. Survey for intestinal parasites in Belize, Central America. Southeast Asian J Trop Med Public Health. 2004; 35(3): 506-511.
- Blumenthal UJ, Cifuentes E, Benner S, Quigley M, Ruiz-Palacios G. The risk of enteric infections associated with wasterwater reuse: the effect of season and degree of storage of wastewater. Trans R Soc Trop Med Hyg, 2001; 95: 131-137.
- Cifuentes E, Suárez L, Espinosa M, Juárez-Figueroa L, Martínez-Palomo A. Risk of Giardia intestinalis infection in children from an artificially recharged groundwater area in Mexico City. Am J Trop Med Hyg, 2004; 71: 65-70.
- World Health Organitzation. The Millennium Development goals and deworming. Report of the third global meeting of the partners for parasite control: deworming for health and development, 29-30 November 2004, World Health Organization, Geneva, 2005.

