Biochemical Differences Between High and Low Dose Methadone Clients on Stable Maintenance Therapy
Jeffrey C Eichhorst1, Adam T Clay2, Jon F Herriot3, Wilna Wildenboer2, Denis C Lehotay4, Tanya E S Dahms5,*
1Roy Romanow Provincial Laboratory, 5 Research Dr, Canada
2Saskatchewan Health Authority, Regina Area, Canada
3Wellesley Health Center, Toronto, Canada
4Department of Pathology and Molecular Medicine, Queens University, Canada
5The Department of Chemistry and Biochemistry, University of Regina, Canada
Received Date: 14/09/2021; Published Date: 08/10/2021
*Corresponding author: Dr. Tanya E S Dahms, Ph. D. The Department of Chemistry and Biochemistry, University of Regina, 3737 Wascana Parkway, Regina, SK, S4S 0A2, Canada
Abstract
The methadone dose required to prevent withdrawal symptoms in chronic methadone users varies dramatically from 5 to 300 mg/day, which could be attributed to a number of biochemical factors, including human µ-opioid receptor (hMOR) density, down-stream desensitization, concurrent drug use or P-glycoprotein (P-gp) expression, or other, possibly genetic factors. Here we describe some biochemical changes of patients taking higher versus lower doses in chronic methadone maintenance therapy (MMT). hMOR density was measured in leukocytes from methadone-maintained patients receiving lower dose (<100 mg/ day) or higher dose (≥100 mg/day) and naive subjects by flow cytometry. Desensitization of receptor signaling was examined by measuring in vivo and attenuated cyclic AMP (cAMP) levels in response to increasing methadone concentrations. P-gp expression and the presence of other drugs were measured using validated methods. In leukocytes, hMOR density did not vary between dosing groups but baseline cAMP levels were significantly lower (p < 0.001) in higher-dose patients (n = 12) than in lower-dose patients (n = 22). Exposure to increasing concentrations of methadone showed a dose-dependent cAMP reduction in naïve leukocytes, but no impact on cAMP levels for those of lower- or higher-dose methadone patients. P-gp levels did not correlate with dose, and concurrent drug use was common. Methadone dose did not correlate with either hMOR density or P-gp levels, but baseline cAMP levels were significantly lower in higher-dose compared to lower-dose patients. Chronic methadone treatment abolished the dose-related reduction in cAMP in vitro in lymphocytes, indicating desensitization. Concurrent drug use may influence dosing requirements. For human subjects, cAMP is a potential biomarker for methadone dosing.
Keywords: Cyclic AMP; Drugs of abuse; Human mu opioid receptor; Methadone maintenance therapy; P-glycoprotein
Introduction
Methadone, a synthetic human µ-opioid receptor (hMOR) agonist, is used for the management of pain as well as a maintenance treatment drug for opioid dependent patients (Ahmad T, 2018; Dinis-Oliveira, 2016) [1,2]. It is metabolized by by the P450 enzymes CYP1A2, CYP2B6 and CYP2C1 in the liver. Methadone metabolites are not pharmacologically active (ED., 2017; Fareed et al., 2010; Garrido & Troconiz, 1999) [3-5]. Methadone dosing is an issue of debate among clinicians in methadone maintenance treatment (MMT) programs (Fareed et al., 2010) [4]. The NIH guideline for proper methadone dosing is at least 60 mg/day, yet 14% of MMT patients receive < 40 mg/day (Fareed et al., 2010) [4]. While most patients function on 60 to 100 mg/day, other research suggests that methadone doses in the range of 120 – 150 mg/day are more effective in reducing heroin self-administration (Fareed et al., 2009) [6]. Approximately 22% of the 1400 MMT patients in a Colorado study were on doses >100 mg/day, with some as high as 300 mg/day (Krantz et al., 2002; D'Aunno et al., 2019; Prommer, 2006) [7-9].
The P450 enzymes are responsible for the conversion of methadone into its primary metabolite, 2-Ethylidene-1,5-Dimethyl-3,3-Diphenylpyrrolidine (EDDP), and affect the kinetics of methadone treatment (Fonseca et al., 2011; Stefano et al., 2009; Wang et al., 2013) [10-12]. However, there is no correlation between the optimal MMT dose and the rate of methadone metabolism or the bioavailability of the biologically active enantiomer of methadone (Leavitt et al., 2000) [13]. Similarly, methadone concentration versus response relationship is quite variable (Chalabianloo et al., 2019; Leavitt et al., 2000; Maxwell & Shinderman, 2002) [13,14,15]. Currently there is no standardized scientific method to determine optimal dose in MMT, and the molecular mechanisms that contribute to variability in dose are not yet fully understood.
Recent studies suggest that genetic factors could be partially responsible for different dose requirements in patients on MMT. Polymorphisms in the opioid receptor delta 1 (OPRD1) maybe associated with methadone dose in patients on MMT (Li et al., 2008) [16]. Single nucleotide polymorphisms (SNP’s) in APBB2 have been shown to correlate with higher incidence of concurrent amphetamine use (Lee et al., 2013) [17]. Heroin and methadone act primarily on the mu-opioid receptor hMOR (Zhang et al., 1998; Zhang et al., 2020) [18,19], while the kappa opioid receptor (hKOR) gene (OPRK1) is critically involved in abstinence and remission. hKOR SNP’s have also been associated with differences in methadone dosage (Zhang et al., 2020) [19]. DNA methylation in two independent cohorts has been shown to be associated with different daily doses, suggesting that these molecular pathways may influence individual dose variability (Marie-Claire et al., 2017) [20]. Another study found that different SNPs in OPRM1, which codes for hMOR, were associated with different methadone dose requirements in African Americans, with each minor allele corresponding to an additional ~20 mg per day of methadone required to prevent withdrawal symptoms (Smith et al., 2017) [21]. In a recent study of Chinese methadone treated patients four SNP’s were shown to be associated with maximum dosage in MMT. These SNP’s were on opiodergic receptors, the cannabinoid receptor COMT, the methadone metabolizing enzymes CYP2B6, and on TPH2, which is a tryptophan metabolizing enzyme (Duan et al., 2020; Mouly et al., 2015) [22,23].
Several studies have shown cellular change in response to opioid use. For example, there are different cyclic AMP (cAMP) and protein kinase C levels in tolerant and opioid naive patients (Hull et al., 2010; Stefano et al., 2009) [11,24]. cAMP, an important second messenger in signaling, is influenced when methadone binds to hMOR. Prolonged exposure to opioids causes an up-regulation of cAMP, and gives rise to moderate levels of tolerance (Bie et al., 2005; Colvin et al., 2019) [25,26]. As a result, the variability between patients on lower dose and higher dose MMT could relate to variable hMOR expression (Bailey et al., 2006) [27], differences in the sensitivity of the receptor, or modifications in the downstream signaling pathways, which can be measured by an attenuated cAMP response.
The P-glycoprotein multidrug efflux pump (P-gp) has been shown to play a role in the methadone analgesic effect (Betts et al., 2016; Meaden et al., 2002) [28,29]. Defining the preexisting level of P-gp expression and activity in peripheral lymphocytes may be required to understand how P-gp alters therapeutic response (Vasquez et al., 2005) [30]. It is possible that P-gp plays a role in methadone dosing requirements.
In MMT, concurrent use of other opioid drugs by patients is common, so reasons for dosage variability may also be attributed to drug-drug interactions, or a combination of pharmacokinetic/ pharmacodynamics (PK/PD) parameters (Boulton et al., 2001; Volpe et al., 2018) [31,32], hence it is important to consider the influence of other drugs in determining dosage requirements. hMOR density is greatest in neuronal cells of the brain and the central nervous system, but in vivo experimentation with human patients is difficult because it would require sampling spinal fluid or brain tissue.
hMOR is present in cells of the immune system (Sibinga & Goldstein, 1988) [33], specifically lymphocytes, monocytes and granulocytes (McCarthy et al., 2001) [34]. There is evidence that opioid receptor density and mRNA for mu- and delta opioid receptors is reduced in leukocytes suggesting that hMOR expression in leukocytes maybe related to receptor density in neuronal cells (Beck et al., 2002; Toskulkao et al., 2010) [35,36], and maybe used as a marker for down-regulation of G-protein coupled opioid receptor expression. Since cyclic AMP is the second messenger for G-protein mediated opioid receptor signalling, we posulated that levels of cAMP in blood cells could provide further support for the leukocyte model (Vousooghi et al., 2009) [37]. Measuring levels of hMOR and cAMP in leukocytes offers a convenient and practical way to assess drug dosing and its effect on receptor density and downstream signaling in human patients. This study used a leukocyte model to detect biomarkers that might provide insights into the mechanisms that lead to the requirements for higher or lower methadone doses (higher dose is defined as ≥100 mg/day versus lower dose <100 mng/day) to prevent withdrawal symptoms by assessing key factors such as hMOR expression, cAMP levels, P-gp expression and concurrent drug use. These markers were selected to detect possible changes in the signal transduction mechanisms of opioid intake, and to detect possible changes in methadone metabolism or availability.
Material and Methods
All experimental analytical work was performed at the Saskatchewan Disease Control Laboratory and the Pasqua Hospital in Regina, Saskatchewan, Canada. Ethics approval (Bio # 05-150) was obtained for this research on a yearly basis from the University of Saskatchewan Biomedical Research Ethics Board.
Materials and supplies
Dextrose was purchased from Becton Dickinson (Sparks, MD, USA), while dextran, sodium chloride, citric acid, sodium citrate·2H2O, [D-Ala2, N-Me-Phe4, Gly5-ol]-Enkephalin acetate salt (DAMGO), forskolin and epinephrine were purchased from Sigma-Aldrich (Oakville, ON, Canada). Naloxone –FITC was purchased from Invitrogen (Burlington, ON, Canada). Phosphate buffered saline (PBS) buffer was acquired from the Media Preparation Facility at the Saskatchewan Disease Control Laboratory (Regina, SK, Canada). Commercial cAMP immunoassay kits from Assay Designs, a division of Enzo Life Sciences were used (Product # ADI-901-163) and purchased
from MJS Biolynx Inc. (Brockville, Ontario, Canada). P-gp concentrations were measured using a commercial immunoassay kit (Catalog No. CSB-E11709h) purchased from CUSABIO BIOTECH CO., Ltd. (Hubei Province, P. R. China).
Subject selection and blood collection
Twenty-eight study subjects were selected based upon their methadone dose (0.5 – 220 mg/day), their arrival at a methadone clinic in Regina, Saskatchewan, Canada run by one of the authors (WW) (no appointment format) and their willingness to participate. An attempt was made to collect from both genders equally and over as wide an age range (~20-70 yr) as practically possible. All subjects had been on long term (> 6 months) treatment at their respective doses. Subjects were somewhat arbitrarily divided into two groups based on NIH guidelines, consisting of those on a lower dose (< 100 mg/day) and those on a higher dose (> 100 mg/day). The likelyhood of any subject belonging to the higher dose versus lower dose group is not related to any known characteristic or biochemical measure in these study subjects. This study is an attempt to identify possible associations to the biomarkers measured. Blood was collected early each analysis day to facilitate sample processing on the same day. Blood was collected by venipuncture to acquire 5-8 mL of whole blood from each patient, in 2 x 5 mL EDTA vacutainer tubes (Becton Dickinson). An aliquot of 200 µL was used for drug of abuse (DOA) screening. Leukocytes were isolated from most of the remaining blood (approximately 4 mL), and used to measure cAMP levels, hMOR density and P-gp expression.
Leukocyte isolation
Dextran sedimentation was used to isolate leukocytes from blood (Boyum, 1968) [38]. Briefly, acid citrate dextrose solution (2.25% w/v dextrose, 2.51% w/v sodium citrate•2H20, 0.73% w/v anhydrous citric acid), dextran (6% w/v) and dextrose (5 % w/v) were prepared in a NaCl solution (0.9% w/v) and mixed in a 3:10:7 volume ratio, respectively, immediately prior to adding an equal volume of blood. The samples were mixed by gentle inversion and allowed to settle for 30 - 45 min at room temperature (RT). When an opaque supernatant separated from a layer containing erythrocytes, the supernatant was removed by pipette and transferred into centrifuge tubes. The opaque supernatants, containing leukocytes and some erythrocyte contamination, were centrifuged (400 × g, 15 min, 4°C), the supernatant removed and the pellet resuspended (0.8 mL, 0.9 % w/v NaCl). Distilled water (2.4 mL) was added to promote the lysis of contaminant erythrocytes and the sample was allowed to stand for 90 seconds. A solution of NaCl (0.58 mL, 5 % w/v) was added to restore isotonicity. The leukocytes were collected by centrifugation (400 × g, 5 min, 4°C) and the supernatant discarded. Lysis and centrifugation were repeated, the pellet resuspended in distilled water (1.5 mL, 90 s), and NaCl (0.36 mL, 5 %) added. Finally, the pellet was collected by centrifugation (400 × g; 5 min, 4 °C), stored on ice, resuspended in appropriate buffer and used immediately or stored at -70°C.
hMOR density by flow cytometry
A modified flow cytometry method, used to quantitate hMOR density, measured the percentage of leukocytes labeled with the fluorescently coupled opioid receptor ligand, naloxoneFITC (Lang et al., 1996; Toskulkao et al., 2010) [36,39]. Briefly, one leukocyte pellet was suspended in 3 mL PBS buffer and 200 µL aliquots of the suspension pipetted into 12 x 75 mm polystyrene flow cytometry tubes with 30 µL PBS buffer (background fluorescence, BF, and total fluorescence, TF) or 32 µM DAMGO in PBS (non-specific fluorescence, NSF). Tubes were vortexed for 15 s, incubated at RT in the dark for 30 min, vortexed every ten min, centrifuged (400 x g, 3 min) and the supernatant decanted. For TF and NSF, 30 µL of 8 µM naloxone-FITC in PBS was added, and for BF, 30 µL of PBS buffer, each followed by mixing with vortex. Samples were incubated (RT, 30 min) in the dark, vortexed every 10 min, centrifuged (400 x g, 3 min), the supernatant decanted and the wash step repeated three times. Finally, cells were resuspended in 0.5 mL PBS buffer for flow cytometric analysis. Leukocytes were diluted to approximately 1 x106 cells/mL (Beckman Coulter UNICEL DXH 800 Hematology analyzer) and introduced into a FC 500 flow cytometer equipped with CXP version 2.2 software (Beckman Coulter). Dot plot resolution was optimized, voltages and gains adjusted for forward and side scatter (Toskulkao et al., 2010) [36] to identify the 3 leukocyte populations of interest (monocytes, granulocytes and lymphocytes). Fluorophores were excited using a 20mW argon laser (λex = 488 nm), emission intensities detected at 510 – 540 nm, and data analyzed using the CXP cytometer software. Percent fluorescence resulting from hMOR-specific binding was calculated as follows:
[(TF – BF) – (NSF – BF)]x 100%;
(TF – BF)
cAMP Biochemical Analysis
The cAMP Complete Enzyme-Linked Immunosorbent Assay (ELISA) kit, a competitive immunoassay that quantifies cAMP in cells and tissue, measured cAMP in leukocytes according to the manufacture’s specifications. Maximal production of cAMP was tested by the addition of forskolin, a known adenylyl cyclase activator. Leukocyte viability and cAMP second messenger signaling were verified by treating leukocytes with epinephrine to measure cAMP levels in the context of the β-adrenergic receptor pathway. Cell isolates, 200 µL, were incubated with 50 µL of 20 µM epinephrine, with time zero representing in vivo concentrations of cAMP. To test the impact of methadone, leukocyte isolates from MMT and control patients were incubated for 60 min with increasing doses of methadone for dose response curves.
Total P-glycoprotein levels in leukocyte isolates
P-gp was measured using a commercial immunoassay kit for the in vitro quantitation of human P-gp in serum, plasma and other biological fluids. This assay has no significant cross-reactivity or interference, and a limit of detection of 0.39 ng/ mL human P-gp. Standards, purified leukocyte samples and controls were measured in duplicate. Standard curves were generated (1.56, 3.12, 6.25, 12.5, 25, 50 and 100 ng/mL of P-gp) and fit with a four-parameter logistic curve as per the manufacture’s guidelines. Optical densities were determined on a Wallac Victor-2 automated plate reader (Perkin Elmer Life Sciences, Turku, Finland) using Multi-Calc software, with unknown concentrations of P-gp in leukocyte samples calculated from the standard curve.
LC-MS/MS analysis of drugs and metabolites
A 200 µL aliquot of blood was analyzed using a validated tandem mass spectrometry method to detect and quantify common drugs of abuse (Eichhorst et al., 2009) [40].
Statistical Analysis
All statistical analyses were conducted with SPSS version 22 (SPSS Inc., Chicago, IL). Continuous variables were summarized as means and standard deviations, and categorical variables expressed as counts and percentages. The difference between the lower (<100 mg/day) and higher dose group (≥100 mg/day) (Fareed et al., 2009) [6] were assessed with an independent Student’s t-test (continuous variables) and Chisquared or Fisher exact test (dichotomous variables). Spearman correlations were used to test the relationships between dose (as a continuous variable), and cAMP, P-gp and receptor density, using an Bonferroni-adjusted alpha value of 0.004 for significance to account for multiple comparisions.
Results
hMOR density
Leukocytes isolated from subject blood were analyzed by flow cytometry for hMOR density, with results presented in Figure S1 and Table S1. Reproducible receptor density from naïve subjects was below assay detection limits. There was no significant correlation between methadone dose and hMOR density for any of the three leukocyte types (Figure S2) or difference between lower- and higher-dose groups (Table 1), hence no measures of associations were apparent between methadone dose and hMOR density.
Table 1: Patient demographics, receptor density, cAMP and Pgp levels in study participants.
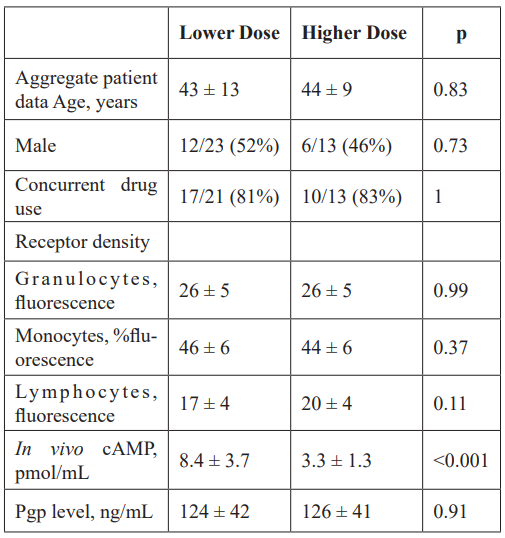
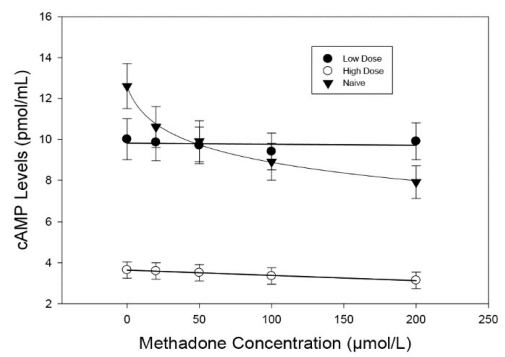
Figure 1: Plot of “mean” cAMP levels as a function of methadone concentration for of naïve, lower dose subjects and higher dose subjects (shown as dose response curves). In contrast to methadone prescribed patients, naïve subjects (n=6) had a significant dose response relationship with logarithmic changes in cAMP levels when exposed to increasing methadone dose.
cAMP and P-gp levels
The non-acetylated version of the cAMP ELISA had inter-assay precision better than quoted. Cells treated with epinephrine resulted in increased cAMP levels, demonstrating that leukocyte isolates were still viable (Figure S3). Viability was confirmed by flow cytometric analysis of cell isolates with propidium iodide (data not shown). Thus, cells would have been receptive to methadone treatment for dose response curves, and subsequent measurement of cAMP. Leukocyte samples thawed from frozen were viable and those analyzed for cAMP levels were reanalyzed for cAMP after several freeze-thaw cycles, showing no significant change in cAMP levels (data not shown). cAMP levels were significantly different between patient groups (Table 1). In vivo cAMP values, estimated from in vitro cAMP measurements, were used to construct dose response curves to methadone, showing very little dose – response for both lowerand higher-dose patients exposed to methadone, unlike naïve subjects (Figure 1; Figure S4). It is known that chronic methadone dosing creates a state of tolerance or desensitization. Our data clearly indicates that in vivo cAMP levels are significantly higher in lower than in higher chronic dosing with methadone, a nover observation. In vivo cAMP levels were lower (M = 8.4 pmol/mL; 99% CI [6.6, 10.1 pmol/mL]) in higher- dose patients (M = 3.3 pmol/mL; 99% CI [2.4, 4.1 pmol/mL]) than in lower-dose patients. Similarly, there was a strong statistically significant correlation (rs (33) = -.885, p < 0.001) between methadone dose and in vivo cAMP levels (Figure 2). Unlike cAMP, there was no correlation between methadone dose and P-gp (Table 1), with no significant relationship between P-gp levels expressed on peripheral leukocytes and methadone dosing requirements (Figure S5).
Table S1: Subject dose and receptor density (percent fluorescence) on leukocytes isolated from blood samples.
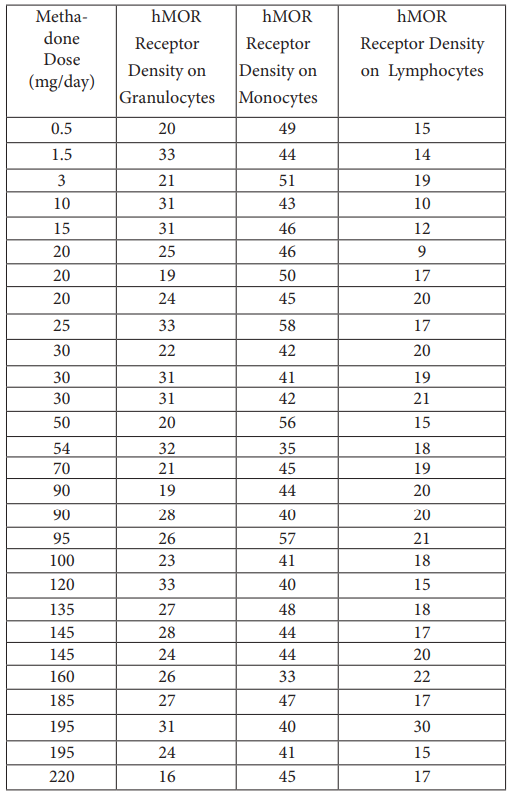

Figure 2: In-vivo cAMP level as a function of methadone dose in all subjects.
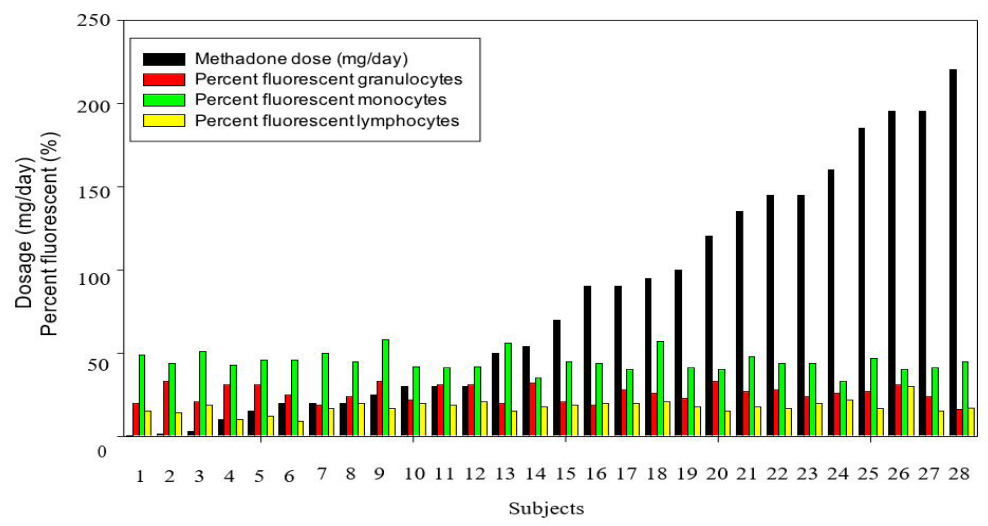
Figure S1: Methadone dose and hMOR expression levels on granulocytes, monocytes and lymphocytes from flow cytometry experi- ments of subject leukocyte isolates.
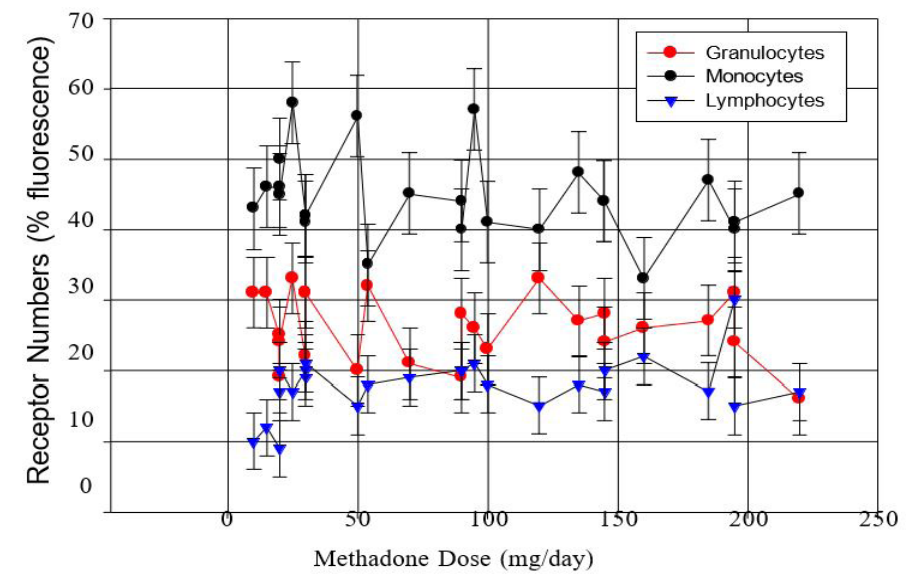
Figure S2: Estimated hMOR density on leukocytes from flow cytometry experiments, expressed as percent fluorescence versus dose of methadone (mg/day).
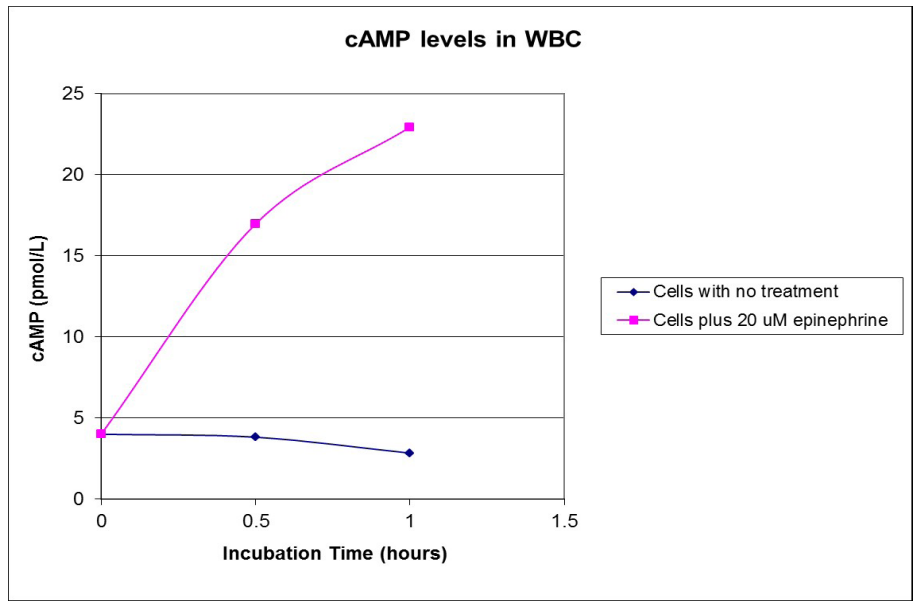
Figure S3: Plot of cAMP level as a function of incubation time with epinephrine used to assess white blood cell viability.

Figure S4: cAMP levels measured from leukocytes versus methadone dose for moderate and high dose subjects (mg/day). Red =lower dose, blue = higher dose, green = high dose subject with highest cAMP values.
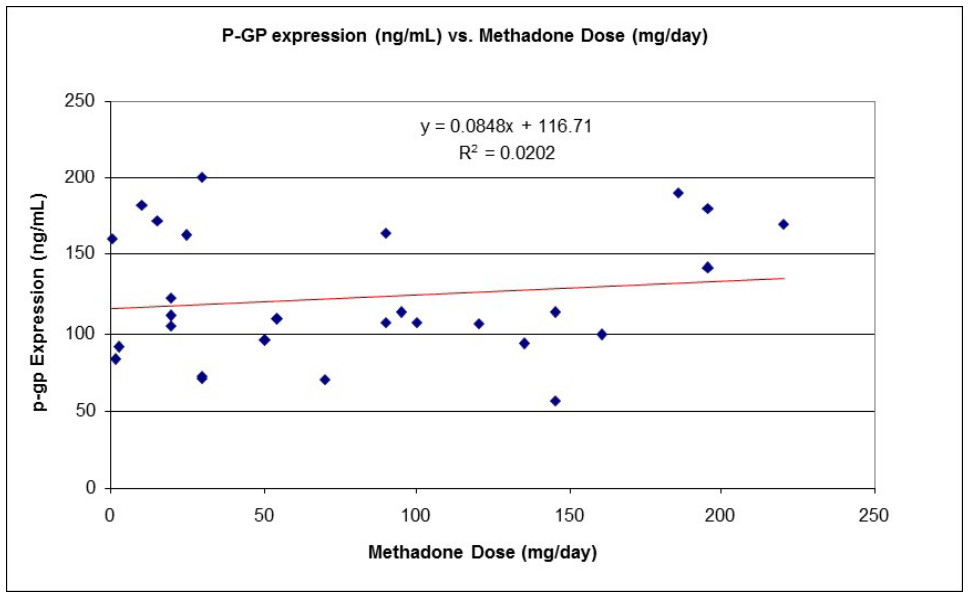
Figure S5: P-gp expression as a function of methadone dose in all subjects (n = 28).
Table S2: Correlation between methadone dose and receptor density, cAMP and Pgp levels.
Table S3: Concurrent drugs of abuse usage among MMT patients.
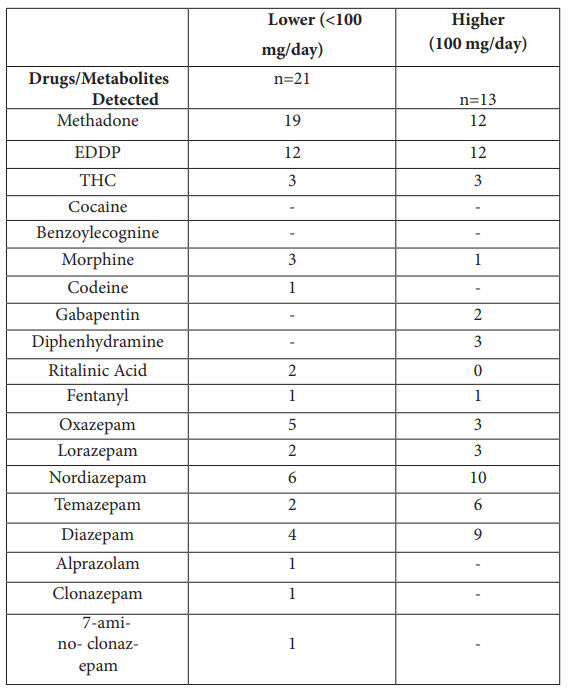
Concurrent drug use
The majority of methadone patients used other drugs during their treatment (Table 1), of which morphine, codeine (metabolized to morphine) and fentanyl are hMOR agonists. Concurrent drug use was assessed with a broad-spectrum screen (Table S3) in which 50 % of MMT subjects tested positive for at least one benzodiazepine or metabolite thereof, and many tested positive for several metabolites. Some of the more promiscuous drug use was associated with the higher dose group, with both gabapetin and diphenhydramine detected. The abuse of morphine agonists was evenly split between lower- and higher-dose subjects, five each, such that comparison of lowerversus higher-dose subjects should not be skewed by hMOR drugs of abuse. Urine samples, normally higher in methadone metabolites (EDDP), were not collected. For subjects on lower methadone doses, blood levels of EDDP are below the detection limits, explaining why half the lower-dose subjects did not test positive for EDDP.
Discussion
hMOR density on leukocytes
The hMOR mediates or acts as a transducer for the action of most clinically important analgesics and opioids, including methadone. Previous studies have suggested that hMOR is responsible (at least in part) for tolerance and dependence (Jordan & Devi, 1998) [41]. There was no significant difference in receptor number based on dosing, demonstrating that dosing requirements for these methadone subjects could not be attributed to differences in hMOR density (Urhan-Kucuk et al., 2011) [42]. Chronically dosed subjects (> 6 months) expressed measureable levels of hMOR receptors on leukocytes. It was not possible to reliably measure hMOR on leukocytes of naïve subjects, suggesting that methadone treatment at any dose induces hMOR expression, consistent with cell culture studies showing that methadone treatment induces hMOR expression (Suzuki et al., 2000) [43]. However, receptor up regulation alone is insufficient to explain dose requirements for individuals, implying that other mechanisms of desensitization such as decoupling of downstream signalling effects, receptor internalization and/or sequestering, other genetic factors, or alterations in G protein signal transduction mechanism, may be responsible for the variable dose requirements (Williams et al., 2001) [44]. Genetic polymorphisms in the gene coding for hMOR have been well described with more than 20 variants identified having amino acid substitutions and altered affinity to various substrates. The overall effect on methadone binding is unclear, although some studies have shown certain variants to have a decreased opioid effect and increased opioid dosage requirements (Waldhoer et al., 2004) [45]. Indeed, SNP’s in the genes coding for the opioid receptors appear to influence the dose of methadone required to prevent withdrawal symptoms (Duan et al., 2020; Lee et al., 2013; Mouly et al., 2015; Smith et al., 2017; Zhang et al., 2020) [19,21,22].
Downstream signaling and variations in cAMP levels
Both tolerance and dependence following methadone treatment are associated with an up-regulation of the second messenger cAMP (Wang et al., 1999) [46]. Since cAMP is an important signalling molecule, cAMP baseline levels and those at increasing doses of methadone were measured in all patients. Chronically treated MMT patients had in vivo baseline cAMP levels that correlated strongly with methadone dose (rs = -.885).
Higher-dosed subjects had lower levels of cAMP than their lower-dosed counterparts (Table 1). The leukocytes of all chronically treated MMT patients showed no cyclic AMP response to exogenous methadone, suggesting tolerance due to prior methadone exposure in vivo, unlike those of naïve subjects with an overall higher baseline level of cAMP. Naïve users had an obvious response to increased levels of methadone, while MMT patients did not (Figure 1). Previous studies on methadone-addicted rats showed tolerance and reduced cAMP levels following acute treatment (Sadava & Mack, 1986) [47]. This is consistent with our results demonstrating the complex role of cAMP-mediated signal transduction in addiction and the development of tolerance. A strong, statistically significant correlation between decreasing cAMP levels and increasing chronic methadone dose of the subjects of this study indicates that higher dosed patients had a lower baseline level of cAMP. The aim of methadone treatment is to prevent withdrawal symptoms. The downstream signalling mechanisms in higherand lower-dose patients are different. Our data indicates that this difference somehow results in a greater reduction of cAMP in higher-dose patients than in lower-dose patients. Both higher-dose and lower-dose patients are given sufficient methadone to prevent withdrawal, but the mechanisms for the difference in the signal transduction pathways are unclear. Our data supports the idea that downstream signalling is a major factor in controlling withdrawal from opioid drugs (Bie et al., 2005) [25], and that dosing with methadone to reduce withdrawal symptoms is mediated through cAMP-mediated mechanisms. The details by which higher or lower in-vivo cAMP levels bring about the reduction of withdrawal in higher- or lower-dose patients’ needs to be investigated in future studies.
P-glycoprotein and methadone dosing
It was conceivable that P-gp expression might play a significant role in methadone PK/PD processes, which could affect dosing requirements. Although the influence of P-gp on PK or PD parameters was not measured directly, P-gp expression was measured with the assumption that increased expression may represent increased influence of PK/PD. The lack of correlation between leukocyte P-gp expression and methadone dose demonstrates that variability in methadone dosing requirements among MMT patients is not linked to P-gp expression. It is however possible that leukocyte levels do not reflect P-gp activity (Vasquez et al., 2005) [30] or levels in other organ systems, for example the blood brain barrier. There are polymorphisms in the MDR1 gene encoding P-gp. Many studies show genotype does not significantly affect methadone dose requirements (Dennis et al., 2014) [48], but others suggest that P-gp variants do impact the outcome of MMT (Buchard et al., 2010; Lee et al., 2013; Yuferov et al., 2010) [17,49,50] and influence drug- drug interactions in patients receiving concomitant drugs that are also P-gp substrates (Hung et al., 2013) [51]. Further studies are required to fully elucidate the role of P-gp in methadone treatment.
Methadone treatment and concurrent drug use
The common use of other drugs increases the likelihood of variability in patient response to methadone and certainly we observe a high subject usage of benzodiazepines, the most frequently detected drug. The benzodiazepine family of drugs are extensively metabolized, with many active metabolites capable of pharmacological activity and the potential for more complex drug-drug interactions (Concheiro et al., 2007) [52].
Our data correlates well with a study from Switzerland, in which the benzodiazepine usage rate amongst methadone patients was 51.5% (Chen et al., 2011) [53], with a worldwide range of 20 – 70% for MMT patients. Of the 101 methadone patients in the Swiss study, 52 (51.5%) were regular users of benzodiazepines and 48 of those received their benzodiazepines by medical prescription. Based on the lack of evidencebased recommendations for benzodiazepine prescription to MMT patients, physicians often find themselves in a dilemma. Not prescribing benzodiazepines during MMT increases the risk and likelihood of patients that are benzodiazepine abusers, not tolerating cessation and dropping out of the program. On the other hand, their prescription means risking continued (Chen et al., 2011) [53] or new dependence on a set of concurrent drugs.
Drugs such as other opioids or narcotics may interact with methadone through the opioid receptors. Buprenorphine, a partial agonist, or mixed agonist-antagonist analgesics such as nalorphone can displace methadone from receptors and should be avoided in patients undergoing MMT (Li et al., 2008) [16]. A high degree of methadone dose variability and methadone’s relatively narrow therapeutic index are related to metabolism, drug transport and hMOR interaction (Buchard et al., 2010) [49]. All of these phenomena are complex and require consideration by clinicians trying to personalize safe and effective methadone administration. Genetic factors are not the only cause of inter-individual variability and it is important to include other factors such as co-medication, state of health, environmental and biological factors.
Conclusions
Tolerance and adaptations to methadone treatment are difficult parameters to measure. There was no significant difference in human µ opioid receptor expression between higher- and lower-dose MMT patients. Chronic exposure to methadone did not appear to up-regulate hMOR expression in leukocytes, with no significant difference in receptor density in all types of leukocytes amongst lower- versus higher-dosed patients. In general, higher dosed subjects legitimately require higher methadone doses to control withdrawal symptoms. In contrast to the apparent absence of a relationship between P-gp and methadone dose, there is a correlation between cAMP levels in leukocytes and methadone dosing levels. Cells of naïve individuals had the highest cAMP levels, showing a dose-response effect with
increased methadone exposure. Chronically dosed methadone subjects, regardless of dosage level, had no significant response to increased methadone exposure, with the highest dosed subjects displaying the lowest cAMP levels. This research identifies cAMP as a potential biomarker for methadone dosing in human subjects. Future research will examine the mechanisms responsible for these differences in dosing, and its relationship to cAMP.
Reliability is an issue with the patient demographic, making these studies challenging, but data from this study might be used to design and inform a larger study using our validated laboratory methods and the most accessible tissue (blood) with direct application to the clinical setting.
Funding: Funding for this study was provided in part by the University of Saskatchewan, the University of Regina, the Saskatchewan Disease Control Laboratory and a Natural Science and Engineering Research Discovery Grant to TESD (228206).
Declaration of Interest: The authors report no conflicts of interest.
References
- Ahmad TVM, Rankin GO. Effects of cytochrome P450 single nucleotide polymorphisms on methadone metabolism and pharmacodynamics. Biochem Pharmacol. 2018; 153: 196-204.
- Dinis-Oliveira R J. Metabolomics of methadone: clinical and forensic toxicological implications and variability of dose response. Drug Metab Rev, 2016; 48(4): 568- 501.
- ED K. Current Concepts in Methadone Metabolism and Transport. Clin. pharmacol. drug dev, 2017; 6(2): 125-134.
- Fareed A, Casarella J, Amar R, Vayalapalli S, Drexler K. Methadone maintenance dosing guideline for opioid dependence, a literature review. J Addict Dis, 2010; 29(1): 1-14.
- Garrido MJ, Troconiz IF. Methadone: a review of its pharmacokinetic/pharmacodynamic properties. J Pharmacol Toxicol Methods, 1999; 42(2): 61- 530.
- Fareed A, Casarella J, Roberts M, Sleboda M, Amar R, Vayalapalli S. High dose versus moderate dose methadone maintenance: is there a better outcome? J Addict Dis, 2009; 28(4): 399-405.
- Krantz MJ, Lewkowiez L, Hays H, Woodroffe MA, Robertson AD, Mehler PS. Torsade de pointes associated with very-highdose methadone. Ann Intern Med, 2002; 137(6): 501-504.
- D’Aunno T, Park SE, Pollack HA. (Evidence-based treatment for opioid use disorders: A national study of methadone dose levels, 2011-2017. Journal of Substance Abuse Treatment, 2019; 96: 18-22.
- Prommer E. Rotating methadone to other opioids: a lesson in the mechanisms of opioid tolerance and opioid-induced pain. J Palliat Med, 2006; 9(2): 488-493.
- Fonseca F, de la Torre R, Diaz L, Pastor A, Cuyas E, Pizarro N, et al. Contribution of cytochrome P450 and ABCB1 genetic variability on methadone pharmacokinetics, dose requirements, and response. 526 PLoS One, 2011; 6(5): e19527.
- Stefano GB, Kream RM, Esch T. Revisiting tolerance from the endogenous morphine perspective. Med Sci Monit, 2009; 15(9): RA189-198.
- Wang SC, Ho IK, Tsou HH, Liu SW, Hsiao CF, Chen CH, et al. Functional genetic polymorphisms in CYP2C19 gene in relation to cardiac side effects and treatment dose in a methadone maintenance cohort. OMICS, 2013; 17(10): 519-526.
- Leavitt SB, Shinderman M, Maxwell S, Eap CB, Paris P. When “enough” is not enough: new perspectives on optimal methadone maintenance dose? [Review] [32 refs]. Mount Sinai Journal of Medicine, 2000; 67(5-6): 404-411.
- Chalabianloo F, Westin AA, Skogvoll E, Bramness JG, Spigset O. Methadone serum concentrations and influencing factors: A naturalistic observational study. Psychopharmacology (Berl), 2019; 236(11): 3159-3167.
- Maxwell S, Shinderman MS. Optimizing long-term response to methadone maintenance treatment: a 152-week follow-up using higher-dose methadone. J Addict Dis, 2002; 21(3): 1-12.
- Li Y, Kantelip JP, Gerritsen-van Schieveen P, Davani S. Interindividual variability of methadone response: impact of genetic polymorphism. Mol Diagn Ther, 2008; 12(2), 109-124.
- Lee HY, Li JH, Sheu YL, Tang HP, Chang WC, Tang TC, et al. Moving toward personalized medicine in the methadone maintenance treatment program: a pilot study on the evaluation of treatment responses in Taiwan. Biomed Res Int, 2013; 741403.
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, et al. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proceedings of the National Academy of Sciences of the United States of America, 1998; 95(12): 7157-7162.
- Zhang Q, Shi M, Tang H, Zhong H, Lu X. kappa Opioid Receptor 1 Single Nucleotide Polymorphisms were Associated with the Methadone Dosage. Genetic Resting and Molecular Biomarkers,2020; 24(1): 17-23.
- Marie-Claire C, Jourdaine C, Lepine JP, Bellivier F, Bloch V, Vorspan F. Pharmacoepigenomics of opiates and methadone maintenance treatment: current data and perspectives. Pharmacogenomics, 2017; 18(14): 1359-1372.
- Smith AH, Jensen KP, Li J, Nunez Y, Farrer LA, Hakonarson H, et al. Genome-wide association study of therapeutic opioid dosing identifies a novel locus upstream of OPRM1. Mol Psychiatry, 2017; 22(3): 346-352.
- Duan L, Li X, Yan J, Chen Y, Luo R, Zhang Q, et al. Association of COMT Gene Polymorphisms with Response to Methadone Maintenance Treatment Among Chinese Opioid-Dependent Patients. Genetic Testing and Molecular Biomarkers, 2020; 24(6): 364-369.
- Mouly S, Bloch V, Peoc’h K, Houze P, Labat L, Ksouda K, et al. Methadone dose in heroin-dependent patients: role of clinical factors, comedications, genetic polymorphisms and enzyme activity. Br J Clin Pharmacol, 2015; 79(6): 967-977.
- Hull LC, Llorente J, Gabra BH, Smith FL, Kelly E, Bailey C, et al. The effect of protein kinase C and G protein-coupled receptor kinase inhibition on tolerance induced by mu-opioid agonists of different effica Pharmacol Exp Ther, 2010; 332(3): 1127-1135.
- Bie B, Peng Y, Zhang Y, Pan ZZ. cAMP-mediated mechanisms for pain sensitization during opioid withdrawal. J Neurosci, 2005; 25(15): 3824-3832.
- Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet, 2019; 393(10180): 1558-1568.
- Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. How important is protein kinase C in mu-opioid receptor desensitization and morphine tolerance? Trends Pharmacol Sci, 2006; 27: 558-565.
- Betts KS, Chan G, McIlwraith F, Dietze P, Whittaker E, Burns L, et al. Differences in polysubstance use patterns and drug-related outcomes between people who inject drugs receiving and not receiving opioid substitution therapies. Addiction, 2016; 111(7): 1214-1223.
- Meaden ER, Hoggard PG, Khoo SH, Back DJ. Determination of P-gp and MRP1 expression and function in peripheral blood mononuclear cells in vivo. J Immunol Methods, 2002; 262(1-2): 159-165.
- Vasquez EM, Petrenko Y, Jacobssen V, Sifontis NM, Testa G, Sankary H, et al. An assessment of P-glycoprotein expression and activity in peripheral blood lymphocytes of transplant candidates. Transplant Proc, 2005; 37(1): 175-177.
- Boulton DW, Arnaud P, Devane CL. Pharmacokinetics and pharmacodynamics of methadone enantiomers after a single oral dose of racemate. Clinical Pharmacology & Therapeutics, 2001; 70(1): 48-57.
- Volpe DA, Xu Y, Sahajwalla CG, Younis IR, Patel V. Methadone Metabolism and Drug-Drug Interactions: In Vitro and In Vivo Literature Review. J Pharm Sci, 2018; 107(12): 2983-2991.
- Sibinga NE, Goldstein A. Opioid peptides and opioid receptors in cells of the immune system. Annu Rev Immunol, 1988; 6: 219-249.
- McCarthy L, Szabo I, Nitsche JF, Pintar JE, Rogers TJ. Expression of functional mu-opioid receptors during T cell development. J Neuroimmunol, 2001; 114(1-2): 173-180.
- Beck M, Mirmohammadsadegh A, Franz B, Blanke J, Hengge UR. Opioid receptors on white blood cells: effect of HIV infection and methadone treatment. Pain, 2002; 98(1-2): 187-194.
- Toskulkao T, Pornchai R, Akkarapatumwong V, Vatanatunyakum S, Govitrapong P. Alteration of lymphocyte opioid receptors in methadone maintenance subjects. Neurochem Int, 2010; 56(2): 285-290.
- Vousooghi N, Goodarzi A, Roushanzamir F, Sedaghati T, Zarrindast MR, Noori-Daloii MR. Expression of mu opioid receptor splice variants mRNA in human blood lymphocytes: a peripheral marker for opioid addiction studies. Int Immunopharmacol, 2009; 9(7-8): 1016-1020.
- Boyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scan J Clin Lab Invest Suppl, 1968; 97: 31-50.
- Lang ME, Davison JS, Bates SL, Meddings JB. Opioid receptors on guinea-pig intestinal crypt epithelial cells. J Physiol, 1996; 497(Pt 1): 161-174.
- Eichhorst JC, Etter ML, Rousseaux N, Lehotay DC. Drugs of abuse testing by tandem mass spectrometry: a rapid, simple method to replace immunoassays. 513 Clin Biochem, 2009; 42(15): 1531-1542.
- Jordan B, Devi LA. Molecular mechanisms of opioid receptor signal transduction. Br J Anaesth,1998; 81(1): 12-19.
- Urhan-Kucuk M, Erdal ME, Ozen ME, Kul S, Herken H. Is the dopamine D3 receptor mRNA on blood lymphocytes help to for identification and subtyping of schizophrenia? Molecular Biology Reports, 2011; 38(4): 2569-2572.
- Suzuki S, Miyagi T, Chuang TK, Chuang LF, Doi RH, Chuang RY. Morphine upregulates mu opioid receptors of human and monkey lymphocytes. Biochem Biophys Res Commun, 2000; 279(2): 621-628.
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev, 2001; 81(1): 299-343.
- Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem, 2004; 73: 953-990.
- Wang Z, Bilsky EJ, Wang D, Porreca F, Sadee W. 3-Isobutyl-1-methylxanthine inhibits basal mu-opioid receptor phosphorylation and reverses acute morphine tolerance and dependence in mice. Eur J Pharmacol, 1999; 371(1): 1-9.
- Sadava D, Mack B. The effect of methadone addiction on cyclic nucleotide levels in regions of rat brain. Life Sci, 1986; 39(5): 477-481.
- Dennis BB, Bawor M, Thabane L, Sohani Z, Samaan Z. Impact of ABCB1 and CYP2B6 genetic polymorphisms on methadone metabolism, dose and treatment response in patients with opioid addiction: a systematic review and meta-analysis. PLoS One, 2014; 9(1): e86114.
- Buchard A, Linnet K, Johansen SS, Munkholm J, Fregerslev M, Morling N. Postmortem blood concentrations of R- and S-enantiomers of methadone and EDDP in drug users: influence of co-medication and p-glycoprotein genotype. J ForensicSci, 2010; 55(2): 457-463.
- Yuferov V, Levran O, Proudnikov D, Nielsen DA, Kreek MJ. Search for genetic markers and functional variants involved in the development of opiate and cocaine addiction and treatment. Ann N Y Acad Sci, 2010; 1187: 184-207.
- Hung CC, Chiou MH, Teng YN, Hsieh YW, Huang CL, Lane HY. Functional impact of ABCB1 variants on interactions between P-glycoprotein and methadone. PLoS One, 2013; 8(3): e59419.
- Concheiro M, De Castro A, Quintela O, Cruz A, Lopez-Rivadulla M. Determination of illicit drugs and their metabolites in human urine by liquid chromatography tandem mass spectrometry including relative ion intensity criterion. Journal of Analytical Toxicology, 2007; 31(9): 573-580. 489.
- Chen KW, Berger CC, Forde DP, D’Adamo C, Weintraub E, Gandhi D. Benzodiazepine use and misuse among patients in a methadone program. BMC Psychiatry, 2011.

