Ivermectin Use Associated with Reduced Duration of Covid-19 Febrile Illness in a Community Setting
Muhammad Ishaq Ghauri2, Nasir Ali Afsar1, Muslim Abbas1, Muhammad Shariq Mukarram2,*, Muhammad Yahya Peracha1, Khizra Ishaq1
1Department of Medicine, Jinnah Medical and Dental College, Pakistan
2Department of Medicine, Jinnah Medical College Hospital, Pakistan
Received Date: 14/09/2021; Published Date: 07/10/2021
*Corresponding author: Muhammad Shariq Mukarram, Clinical Instructor, Department of Medicine, Jinnah Medical College Hospital, Karachi, Pakistan
Abstract
Background: SARS-CoV-2 infection (COVID-19) is a potentially lethal disease that may progress into severe respiratory distress syndrome requiring ventilatory support. While azithromycin (AZI) and hydroxychloroquine (HCQ) are considered similar to placebo in COVID-19, other drugs such as ivermectin (IVER), are being repurposed to treat this pandemic. This study was designed to assess the effects of ivermectin on duration of febrile illness and disease outcomes in mild-to-moderate COVID-19 infection in a community setting.
Methods: In this case-control study 95 suspected patients of mild-to-moderate COVID-19 were included. The controls (Group-A) received AZI+HCQ for seven days while the cases (Group-B) received IVER+AZI+HCQ for six days.
Results: A total of 41 patients were in Group-B, while 54 patients were in Group-A. Group-B had consistently and significantly shorter span of fever on days 5, 7, 10 and 14, where the logistic regression showed IVER as the major (Exp B 49·55; p<0·001) underlying factor. The Kaplan-Meier survival analysis showed that Group-A had a prolonged febrile illness (p<0·001).
Conclusions: Ivermectin use is associated with reduced duration of febrile illness in COVID-19 in outpatient setting, thus potentially saving precious lives, reducing direct load on healthcare facilities and preventing high cost of management in a community setting.
Keywords: COVID-19; SARS-CoV-2; Ivermectin; Azithromycin; Hydroxychloroquine
Introduction
World continues to fight against COVID-19 pandemic caused by the novel coronavirus SARS-CoV-2. This unprecedented challenge can only be dealt with rigorous research on potential therapeutic drugs and vaccine development that continues globally to date. SARS-CoV-2 infection (COVID-19) usually presents as a multi-system syndrome comprising of varying combination of fever, cough, dyspnea, gastrointestinal upset, body ache, changes in sensations of smell and taste among other symptoms. Many patients suffering from dyspnea would progress into acute respiratory distress syndrome requiring ventilator support. Laboratory investigation usually shows changes in blood count, increased inflammatory response and tissue damage, as well as hypercoagulable state [1]. Radiological criteria exist to aid in COVID-19 diagnosis [2]. The abrupt progression into a pandemic and concomitant strain on economy as well as healthcare infrastructure has led to uneven distribution of diagnostic investigations such as PCR detection kits lagging behind the disease burden in a large part of the global community. That is why a combination of clinical presentation, as well as non-specific laboratory and radiological investigations are widely being relied upon across the world and accepted by WHO [3]. Being a new disease that is still being understood and treatment being sought, a variety of solutions have been proposed to treat it. Newly diagnosed patients of COVID-19 are often prescribed a combination of azithromycin and hydroxychloroquine in anticipation of favorable therapeutic outcome. However, multiple studies have failed to show a beneficial effect of this combination in treating COVID-19 and are not superior to the placebo [4]. While antiviral drugs such as lopinavir/ritonavir, nelfinavir, remdesivir, favipiravir and ribavirin have been tried with varying success and still being explored, other approach includes modulating the immunological and inflammatory response using corticosteroids 5, interferons 6, and IL-6 pathway inhibitors such as tocilizumab, sarilumab, and siltuximab [5].
Some ant parasitic drugs such as chloroquine, Niclosamide, Nitazoxanide and Ivermectin have also been proposed as potentially useful drugs against COVID-19 [6-9]. While chloroquine or hydroxychloroquine does not seem to be an effective treatment for COVID-19 [10,11], ivermectin has shown promising effects. Recent reports suggesting utility of ivermectin in some viral infections [12] and low viral infection in communities having mass deworm campaigns [13] has also led to use of this drug in COVID-19.
With more than 41 million people affected and 1.1 million deaths globally the tragedy continues. Almost half the burden of morbidity and mortality is seen in the worst three affected countries, USA, India and Brazil alone [14]. With burdened healthcare infrastructure, limited resources and few available treatment choices in developed as well as developing countries, all available options could prove vital not only for local use but also globally. This is highly imperative that while new drug and vaccine development is in progress, existing treatment methods may be repurposed to reduce morbidity and mortality at the community level globally as well as help reduce the extreme pressure on healthcare personnel and infrastructure. Hence, we designed this study to assess the effects of ivermectin on duration of febrile illness and disease outcomes in mild-to-moderate COVID-19 infection in a limited-resource community setting in an effort to maximize the ability of healthcare system to deal with the plethora of COVID-19 cases.
Method and Materials
Patients
This is a case-control study conducted at Jinnah Medical and Dental College (JMDC) Karachi, and Jinnah Medical College Hospital (JMCH), Karachi, Pakistan. The research was conducted in accordance with the Declaration of Helsinki and national and institutional standards. The Ethical Review Committee of Jinnah Medical and Dental College, Karachi approved the study.
All patients who presented at JMCH outpatient clinics with strong clinical suspicion of mild-to-moderate COVID-19 [3,15] between March and August 2020, who were not hospitalized, were included. Briefly, patients presenting with a varying combination of fever, cough, fatigue, anorexia, shortness of breath, myalgia, diarrhea, nausea and vomiting, anosmia or ageusia, but no signs of severe pneumonia, including SpO2 ≥ 90% on room air, were defined as mild-to-moderate COVID-19. The diagnostic investigations included PCR testing for SARS-CoV-2, immunoglobulin titers against SARS-CoV-2, pulmonary radiological features (plain radiography and/or CT scan) blood inflammatory markers, such as CRP, and additional investigations depending upon the presenting clinical picture and comorbidities. Being a developing country, there are financial and logistic constraints along with limited availability of public testing facilities because of which not every patient could be tested for COVID-19 through PCR or antibody titers. Therefore, a strong clinical suspicion remained the major diagnostic parameter as described above [3,15].
The patients who received azithromycin (AZI) 500 mg PO daily and hydroxychloroquine (HCQ) 200 mg PO twice daily, both for seven days, were considered as control group (Group-A) while the cases (Group-B) comprised of all those patients who received ivermectin (IVER) 12 mg PO daily for six days in addition to AZI+HCQ.
Data Collection
The general and clinical data of all patients was collected by a questionnaire which included age, gender, history of recent travel or Covid-19 exposure, and significant medical history. A total of 95 patients were included in the study.
Study outcomes
The primary and secondary outcomes of the study are given in Table-1 [11].
Statistical analysis
The groups were compared using chi-square test or Student’s t-test where applicable. Odds ratios with 95% confidence interval were computed for primary and secondary outcomes. Logistic regression analysis was carried out and survival analysis using Kaplan-Meier curves (log-rank) were conducted. In all analyses, only a p-value <.05 was considered significant.
Table 1: Study Outcomes.

Results
A total of 95 patients, including 36 females (38%) and 59 males (62%) diagnosed with COVID-19 with mild to moderate illness were included in this study, who had received azithromycin and hydroxychloroquine (AZI+HCQ) as part of the treatment. Out of those, 41 (Group-B) had also received ivermectin (IVER+AZI+HCQ), while remaining 54 received AZI+HCQ only (Group-A). Patients living in six out of seven urban districts of Karachi were represented. Since the participating hospital is situated in Karachi East, that district remained the dominant group. While patients were predominantly involved with some sort of public dealing, including healthcare and business profession, a quarter of patients were housewives, suggesting the extent of transmission within community and potentially serious outlook. Majority (n=68; 72%) had no comorbidity.
The baseline characteristics are given in Table-2. The majority of patients presented with fever, body ache, lethargy and respiratory complaints although gastrointestinal symptoms were not uncommon. The total leukocyte count showed significant difference between the two groups; however, no clinical significance could be suggested as the values fell well within normal limits. Loss of weight was also common. The most striking difference was whether both groups differed in presence of fever on day 5, 7, 10 and 14. Group-B had consistently and significantly shorter span of fever at each time-point mentioned above. The odds ratio (OR) suggested that Group-B had much higher odds to have a shorter span of febrile illness. The drugs were well-tolerated in the doses used as shown by lack of any reported side effects.
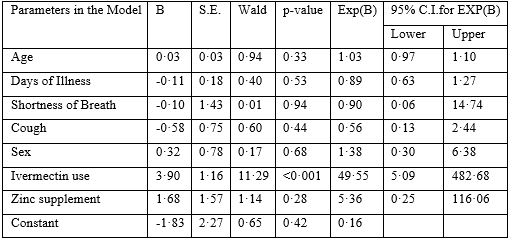
The logistic regression (Table-3) showed that use of ivermectin was the only dominant (Exp(B) 49·55) and significant (p<·001) factor underlying the shorter duration of febrile illness among patients. The use of azithromycin, hydroxychloroquine and zinc was uniform between the groups.
A Kaplan-Meier (K-M) curve with log-rank test (Figure-1) was computed for duration of fever in both groups. The K-M curve showed that Group-A, which did not use ivermectin, was more likely to show a prolonged duration of febrile illness (p<·001) as compared to Group-B, which received ivermectin.
Figure-2, as well as the odds ratio shown in Table-2 shows that the maximum benefit of using ivermectin is seen when prescribed early in the course of illness. If ivermectin is prescribed with a delay of five days or more after the start of febrile illness, its beneficial effect may be reduced.
Table 2: Baseline Characteristics.
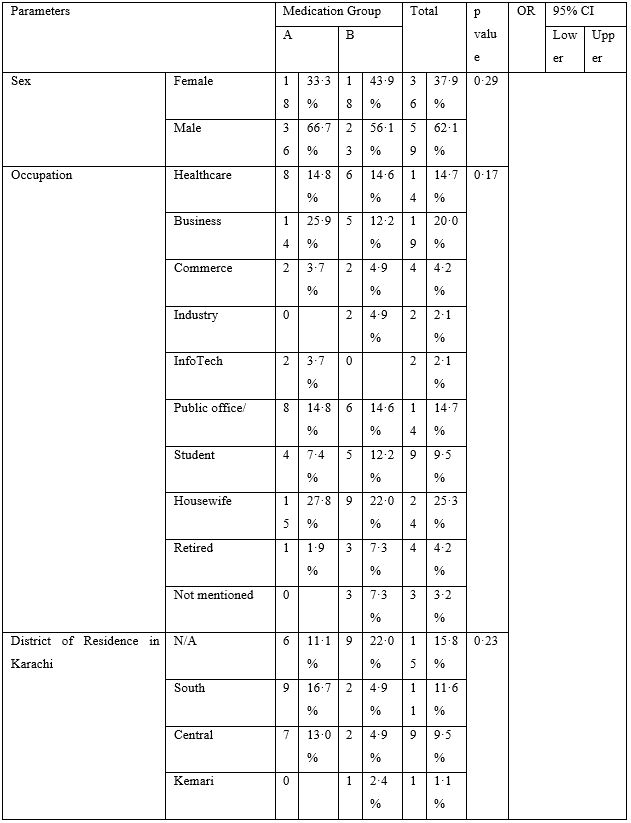
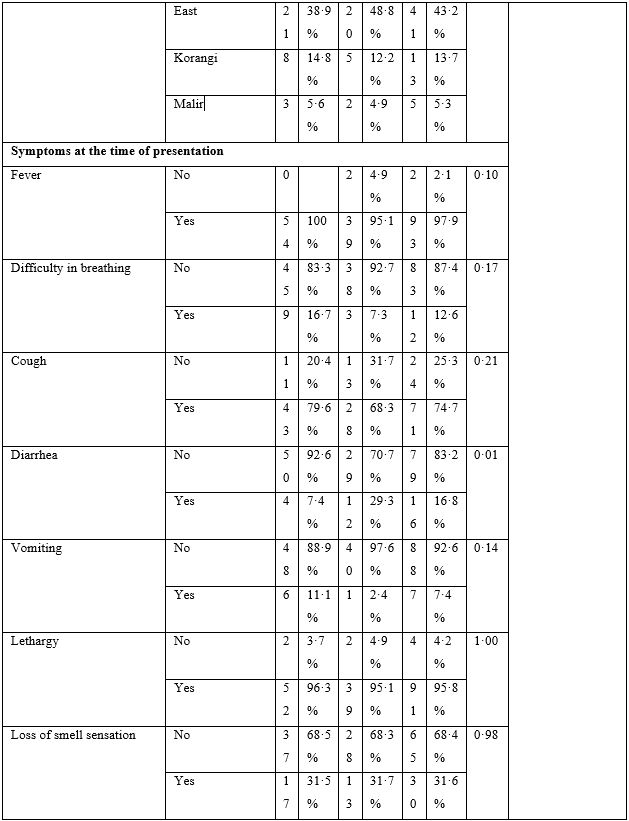
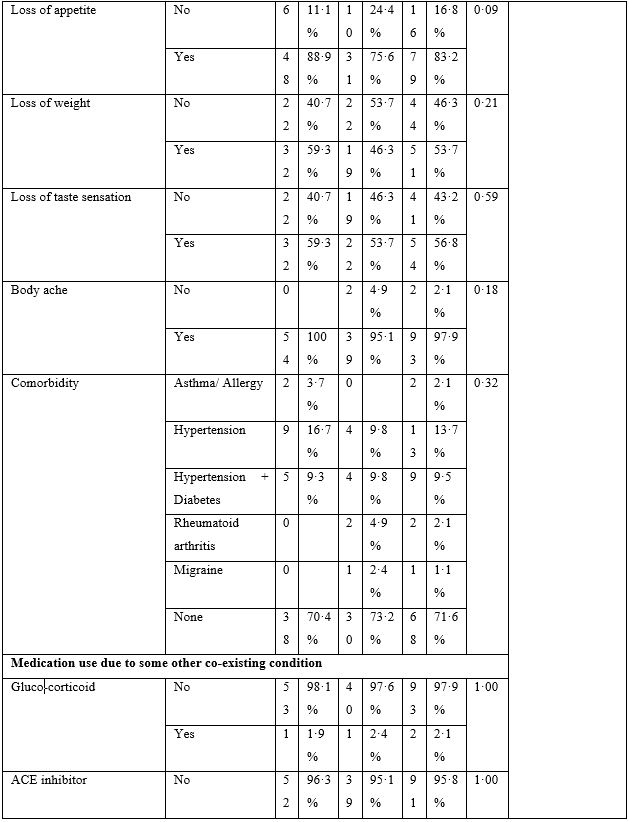
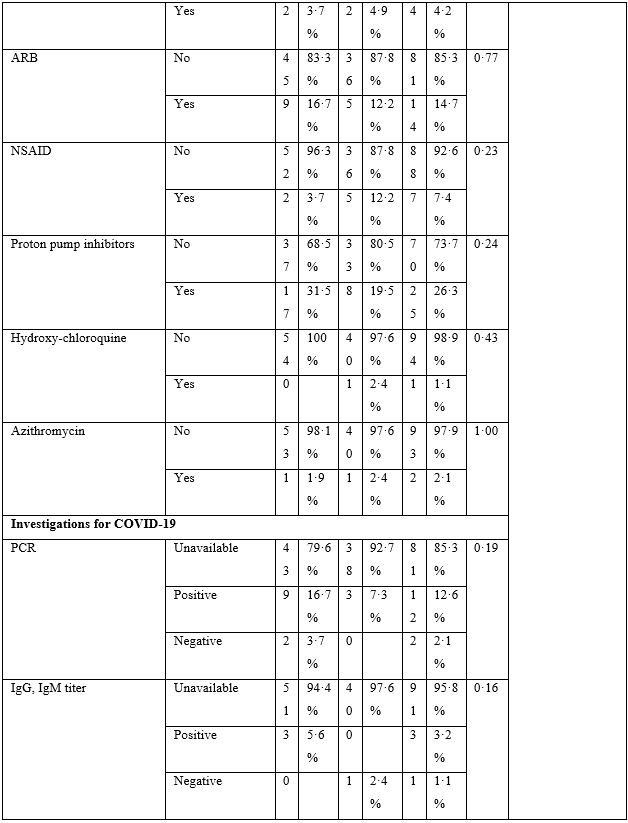
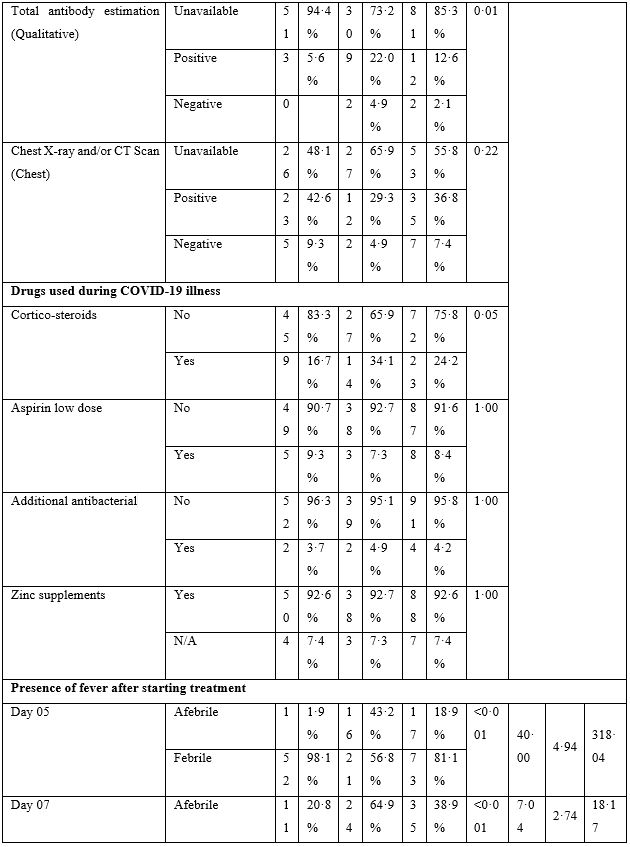

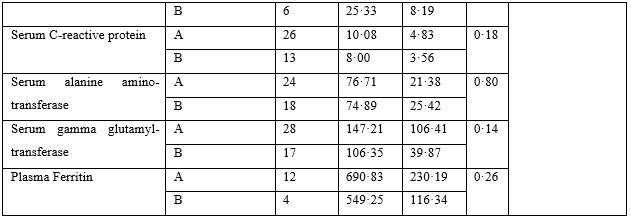
Table 3: Logistic Regression Analysis.

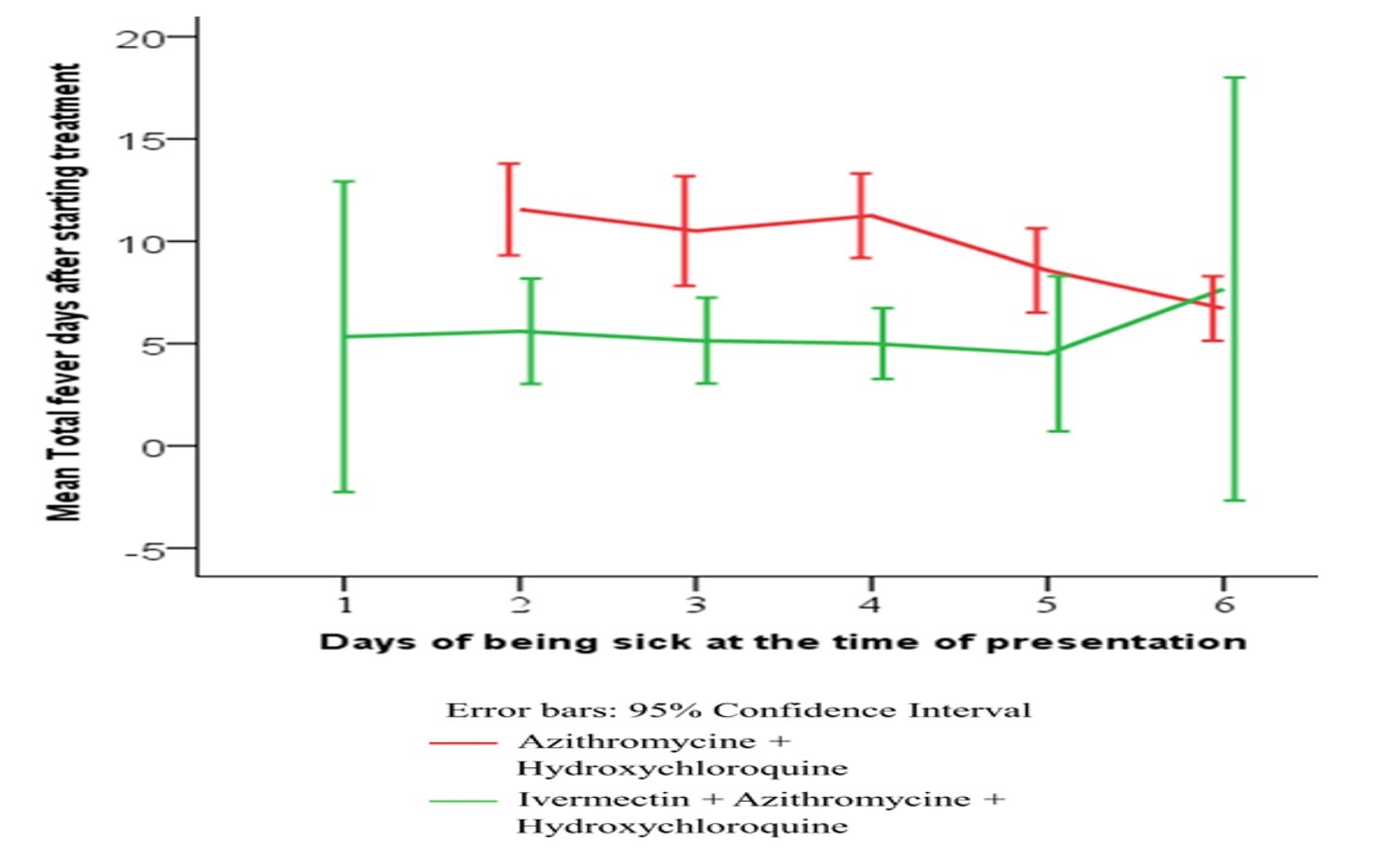
Figure 1: KAPLAN-MEIER ANALYSIS FOR PRIMARY END POINTS (a) AND SECONDARY END POINTS (b).
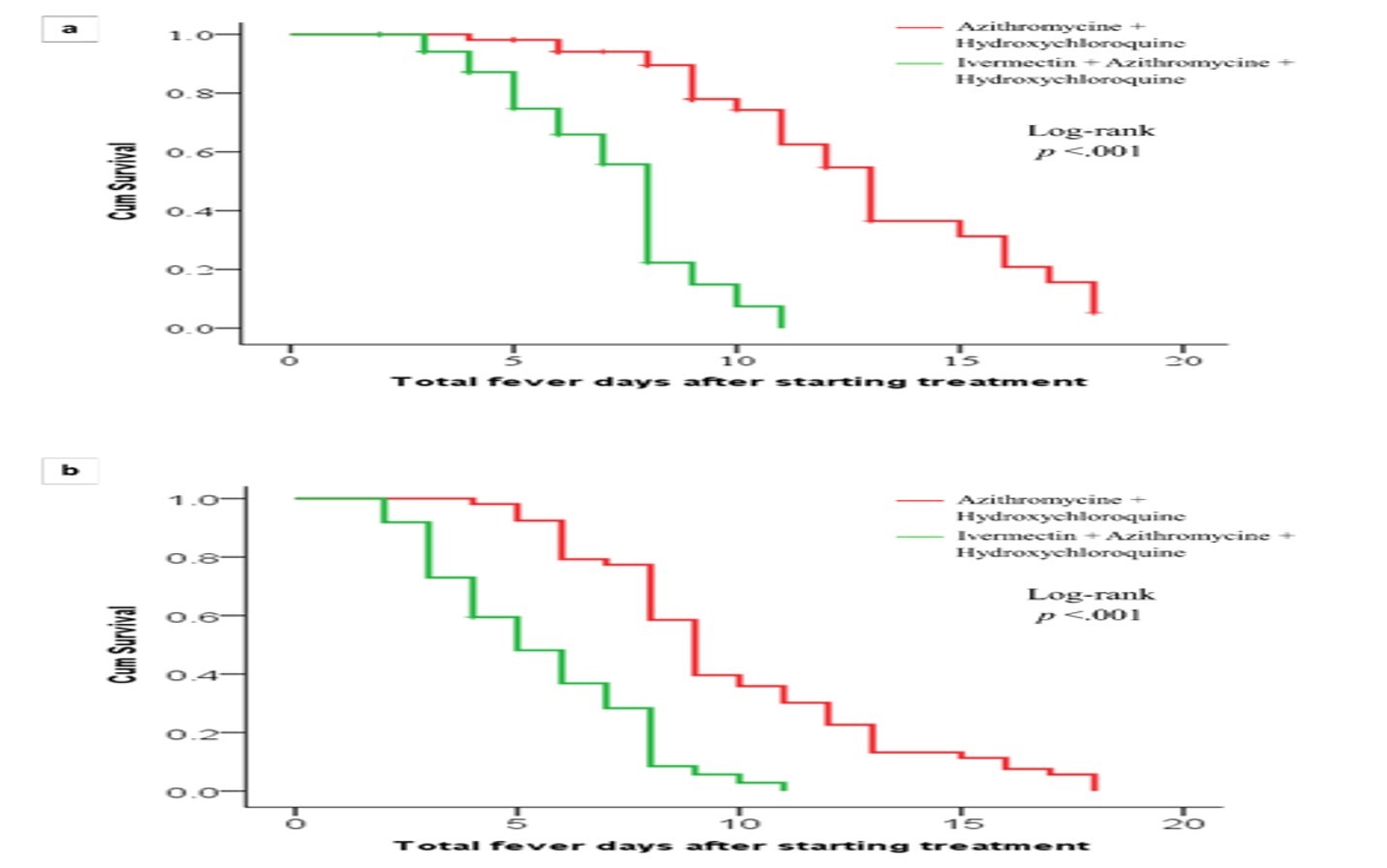
Figure 2: Duration of febrile illness according to the day of treatment commencement.
Supplementary Table:
Logistic Regression Analysis
Discussion
This study shows that ivermectin use is associated with reduced duration of the febrile illness in an outpatient setting, thus promoting early recovery from COVID-19 infection, especially when started early in the course of the illness.
There are several advantages of this study. This study included COVID-19 patients suffering from febrile illness who had presented most frequently on 3rd day of febrile illness (unimodal distribution). The severity of illness and treatment was uniform and suggested an effective, feasible and very affordable method of treatment at community level.
There are some limitations of this study such as a case-control design, and exclusion of severely ill or hospitalized patients. Many patients were diagnosed on the basis of the clinical syndrome rather than the molecular testing, as it was neither widely available nor within reach of the majority of population within the country. Moreover, there are serious concerns over the COVID-19 testing in Pakistan at the community level [16,17] rendering its feasibility in our setting. At places where the molecular testing is considered more standardized, the presence of false positive and false negative results remains a limiting factor within the country creating speculations about the testing itself. RT-PCR is commonly used for a diagnostic test; however, its accuracy is questionable as the absence of the virus cannot be ruled out depending on the basis of PCR alone. The probability of false-negative results is high early in the disease, however, despite a relative reduction of false-negative results by the end of first week of exposure the clinical picture remains the main consideration among those presenting with the COVID-19 infection [18].
Earlier the antimalarial drugs chloroquine and hydroxychloroquine were claimed to possess antiviral activity [19,20], however, such studies were mainly in vitro and needed clinical trials to validate their efficacy and safety in humans. This was even more concerning when chloroquine or hydroxychloroquine in combination with another potentially cardiotoxic drug azithromycin was considered to treat COVID-19 [21,22]. However, recent studies do not support chloroquine or hydroxychloroquine for the treatment of COVID-19 as follows. A comparative observational study involving COVID-19 patients reported that hydroxychloroquine was not superior to control group in terms of worsening of symptoms, respiratory distress, or overall survival, all by the end of 3 weeks of treatment [23]. A clinical trial found no difference in seroconversion rate at 28th day of COVID-19 illness among those receiving hydroxychloroquine compared to control group [10]. Thus, AZI+HCQ does not impart any beneficial effect in COVID-19 and is as good as placebo, albeit with an increased risk of cardiotoxicity.
The adequate safety profile of any drug is pivotal to consider it as a potential drug to treat different diseases and ivermectin certainly possesses this quality. Its safety profile is established in adults on fixed daily doses of up to 36 mg without producing any significant adverse effects [24]. Apart from the antiparasitic effect, ivermectin is known for its antimicrobial, antiviral, and anticancer potential. In this study ivermectin was used in a fixed dose of 12 mg once a day for six days without any reported side effect, thus confirming other similar studies where ivermectin has been used safely in a variety of conditions. With its good safety profile, ability to produce beneficial effects against many RNA and DNA viruses, and effects on various biological mechanisms, they can be considered as a potential treatment of COVID-19 infection.12 In this study, a dose of 12 mg once daily was used for six days leading to a highly feasible cost of under three dollars for the entire course of ivermectin over six days.
Thus, it could be implied that use of ivermectin in COVID-19 could potentially save many patients from adversely progressing to a severer illness thus saving precious lives, reducing direct load on healthcare facilities and preventing high cost of management.
Ivermectin has various effects on different organisms such as regulation of glucose and cholesterol, suppression of cell proliferation, inhibition of viral replication, and reduction in survival in insect vectors [25]. Despite its multiple therapeutic applications, its mechanism of action is still not accurately known. Another beneficial effect of ivermectin is the modulation of the immune system as it decreases immune cell proliferation and cytokine production.25 Both of these effects can be the reason for showing a positive effect in COVID-19 patients as discussed here. Ivermectin inhibits cytokine production as estimated in bronchoalveolar lavage fluids as well as the reduction in serum IgE and IgG1 levels, thus reducing airway inflammation [26]. A recent proteomic investigation, utilizing cell-based assay, suggests a role of ivermectin as an immunomodulator in COVID-19 [27].
There is an evidence that ivermectin also works directly against some viruses, such as HIV1, dengue and yellow fever viruses. Ivermectin is believed to disrupt important cellular processes of HIV1 by inhibiting HIV integrase and non-structural protein 5 polymerase in dengue virus [26]. It is suggested that it also reduces the replication of flaviviruses which cause yellow fever and dengue by inhibiting viral RNA helicase [25]. In an in vitro study, ivermectin showed anti-viral activity in SARS-CoV2 infected Vero/hSLAM cells [9]. RNA viruses rely on importin α/β1 heterodimers for protein translocation during replication, which is blocked by ivermectin [9]. It is postulated that by a similar mechanism it might inhibit replication of SARS-CoV2 [28].
A recent study suggested that ivermectin used in hospitalized patients suffering from severe pulmonary complications would reduce mortality among COVID-19 patients [29]. However, it was a retrospective study, conducted among hospitalized patients many of whom required a combination of various drugs, had multiple confounders requiring statistical adjustments, the administration of ivermectin did not follow a consistent dosing regimen and many of the patients were given ivermectin later in the course of the disease. The primary and secondary end-points were also different as compared to our study. Another study conducted among healthcare personnel suggested a beneficial role of ivermectin in COVID-19 patients [30]. However, it was a cross-sectional study conducted on COVID-19 patients presenting with a wide range of severity including asymptomatic patients as well as severely ill. Further, the study included mixed treatment regimens. In this study, the group taking ivermectin showed a much quicker recovery from the febrile illness at all time-points consistently with an odds ratio being highly significant as discussed below. The most drastic difference was seen within first 5 days, suggesting the potential of ivermectin to bring about a sharp improvement among COVID-19 patients. To complement our observation, logistic regression analysis shows ivermectin being the only significant factor underlying the shorter duration of febrile illness among patients. Similarly, the survival analysis further supports this inference as the Kaplan-Meier curve showed a clear and highly significant advantage of using ivermectin among COVID-19 patients presenting with febrile illness. Interestingly, like other drugs being tried and used for COVID-19, this study suggests that the sooner the treatment is started, the better the magnitude of benefit in terms of a shorter duration of febrile illness. Recently, it has been postulated [31] that hydroxychloroquine may act synergistically with ivermectin to improve recovery in patients infected with SARS-CoV-2, however, we were not able to explore this in our study. An adequately designed randomized trial should provide the evidence for this hypothesis.
Conclusion
Ivermectin used in COVID-19 infection seems to be a safe and well-tolerated drug which could shorten the duration of febrile illness in outpatient setting. The mechanism of action of ivermectin in COVID-19 needs further investigation stepping further in the direction of a direct antiviral as well as immunomodulatory effects. It is recommended that a double-blinded, placebo-controlled trial should be carried out to establish the efficacy of ivermectin in coronavirus infections among patients who have various level of disease severity and ivermectin doses administered.
Acknowledgement
A preprint version of this manuscript titled, “Ivermectin Use Associated with Reduced Duration of COVID-19 Febrile Illness in a Community Setting” is available at SSRN: https://ssrn.com/abstract=3734478 or http://dx.doi.org/10.2139/ssrn.3734478
Funding
This study is not supported or funded by the institution or any other funding body
Conflict of Interest
The authors state that there is no financial or any other conflict of interest in relation to this research.
Authors’ Contribution
NAA and MIG contributed equally. Both contributed in study design, data analysis, writing up the manuscript, overall supervision at their participating institutions respectively, and final approval.
MA contributed in data entry and manuscript writing.
MSM contributed in data collection and manuscript writing.
MYP contributed in data entry and manuscript writing.
KI contributed in data collection.
References
- Pandey A, Nikam AN, Shreya AB, et al. Potential therapeutic targets for combating SARS-CoV-2: Drug repurposing, clinical trials and recent advancements. Life Sci 2020; 256:117883. doi: 10.1016/j.lfs.2020.117883.
- Udugama B, Kadhiresan P, Kozlowski HN, et al. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020; 14: 3822-3835. doi:10.1021/acsnano.0c02624.
- World Health Organization. Clinical management of COVID-19. WHO/2019-nCoV/clinical/2020.5.
- Molina JM, Delaugerre C, Le Goff J, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect 2020; 50: 384. doi: 10.1016/j.medmal.2020.03.006.
- Hozhabri H, Piceci Sparascio F, Sohrabi H, et al. The Global Emergency of Novel Coronavirus (SARS-CoV-2): An Update of the Current Status and Forecasting. Int J Environ Res Public Health 2020; 17: 5648. doi:10.3390/ijerph17165648.
- Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 2020; 395: 1695-1704. doi:10.1016/S0140-6736(20)31042-4.
- Afsar NA. The looming pandemic of COVID-19: What therapeutic options do we have now? J Chin Med Assoc 2020; 83: 508-509. doi:10.1097/JCMA.0000000000000310.
- Kelleni MT. Nitazoxanide/azithromycin combination for COVID-19: A suggested new protocol for early management. Pharmacol Res 2020; 157:104874. doi: 10.1016/j.phrs.2020.104874.
- Caly L, Druce JD, Catton MG, et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 2020; 178:104787. doi: 10.1016/j.antiviral.2020.104787.
- Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 2020; 369:m1849. doi:10.1136/bmj.m1849.
- Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med 2020: doi:10.1056/NEJMoa2019014.
- Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot (Tokyo) 2020; 73: 593-602. doi:10.1038/s41429-020-0336-z
- Wamae CN. Mass Drug Administration and Worms Experience in Africa: Envisage Repurposing Ivermectin for SARS-COV-2. Am J Trop Med Hyg 2020; 103: 10-11. doi:10.4269/ajtmh.20-0295.
- info. Dover, Delaware, USA.
- Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. Morb Mortal Wkly Rep 2020; 69: 759-765. doi:10.15585/mmwr.mm6924e2.
- Anser MK, Yousaf Z, Khan MA, et al. Social and administrative issues related to the COVID-19 pandemic in Pakistan: better late than never. Environ Sci Pollut Res Int 2020; 27: 34567-34573. doi:10.1007/s11356-020-10008-7.
- Atif M, Malik I. Why is Pakistan vulnerable to COVID-19 associated morbidity and mortality? A scoping review. Int J Health Plann Manage 2020; 35: 1041-1054. doi:10.1002/hpm.3016.
- Kucirka LM, Lauer SA, Laeyendecker O, et al. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure. Ann Intern Med, 2020; 173: 262-267. doi:10.7326/M20-1495.
- Savarino A, Di Trani L, Donatelli I, et al. New insights into the antiviral effects of chloroquine. Lancet Infect Dis 2006; 6: 67‐69.
- Biot C, Daher W, Chavain N, et al. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem 2006; 49: 2845-2849. doi:10.1021/jm0601856.
- Gabler E, Keller MH. Prescriptions Surged as Trump Praised Drugs in Coronavirus Fight. NY Times April 25, 2020.
- Sciama Y. Is France’s president fueling the hype over an unproven coronavirus treatment? Science. April 9, 2020.
- Mahévas M, Tran VT, Roumier M, et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ 2020; 369: m1844. doi:10.1136/bmj.m1844.
- Muñoz J, Ballester MR, Antonijoan RM, et al. Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers. PLoS Negl Trop Dis 2018; 12: e0006020. doi: 10.1371/journal.pntd.0006020.
- Laing R, Gillan V, Devaney E. Ivermectin - Old Drug, New Tricks? Trends Parasitol 2017; 33: 463-472. doi:10.1016/j.pt.2017.02.004.
- Crump, A. Ivermectin: enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations. J Antibiot 2017; 70: 495–505. doi:10.1038/ja.2017.11.
- Li N, Zhao L, Zhan X. Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment. J Cell Physiol 2020; 10.1002/jcp.30055. doi:10.1002/jcp.30055.
- Rizzo E. Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action. Naunyn Schmiedebergs Arch Pharmacol 2020; 393: 1153-1156. doi:10.1007/s00210-020-01902-5.
- Rajter JC, Sherman MS, Fatteh N, et al. Use of Ivermectin is Associated with Lower Mortality in Hospitalized Patients with COVID-19 (ICON study). Chest 2020; S0012-3692: 34898-34894. doi: 10.1016/j.chest.2020.10.009
- Malik FT, Ishraquzzaman M, Kalimuddin M, et al. Clinical Presentation, Management and In-Hospital Outcome of Healthcare Personnel With COVID-19 Disease. Cureus 2020; 12: e10004. doi:10.7759/cureus.10004.
- Patrì A, Fabbrocini G. Hydroxychloroquine and ivermectin: A synergistic combination for COVID-19 chemoprophylaxis and treatment? J Am Acad Dermatol 2020; 82: e221. doi: 10.1016/j.jaad.2020.04.017.

