The Role of Probiotics in Clostridioides difficile Disease Severity and Time to Disease Resolution
Stephanie Dym1, Meredith Akerman2, Nicole Fenner3, Burke A Cunha4, Sharon Blum1,*
1Department of Pharmacy, NYU-Winthrop Hospital, Mineola, New York, USA
2Department of Biostatistics, NYU-Winthrop Hospital, Mineola, New York, USA
3Long Island University Arnold and Marie Schwartz College of Pharmacy, Mineola, New York, USA
4Department of infectious Disease NYU-Winthrop Hospital, Mineola, New York, USA
Received Date: 02/09/2021; Published Date: 24/09/2021
*Corresponding author: Sharon Blum, Department of Pharmacy, NYU-Winthrop Hospital, Mineola, New York, USA
Abstract
Background: Clostridioides Difficile Infection (CDI) is the leading cause of hospital-related diarrhea. It accounts for 15,000-30,000 deaths per year in the US and costs approximately $5 billion annually. The Infectious Disease Society of America guidelines have no recommendations regarding probiotic therapy. There is conflicting data regarding its efficacy and research on the role of probiotics in disease severity is lacking. Our primary objectives are to assess the impact of probiotics on CDI severity and disease resolution.
Methods: This was an IRB approved, single-centered, retrospective cohort analysis. Electronic medical records identifying patients diagnosed with CDI in NYU-Winthrop Hospital between 8/1/15-2/28/17 were reviewed. Clostridioides difficile positive patients were allocated into four groups depending on probiotic administration and time of initiation. Patients with a +tcdB gene were included. Patients with missing severity values or time to formed stool data were excluded. The primary outcomes were incidence of severe CDI and time to disease resolution. Chi-square or Fisher’s exact tests were used to compare groups for categorical variables; the Mann-Whitney or Kruskal-Wallis tests were used for continuous data. Time to CDI resolution was analyzed using standard methods of survival analysis. The groups were compared using the log-rank test.
Results: 210 CDI cases were analyzed, 65% of which were severe. 56% of patients were female and median age was 75 years (18.6-97.5). There was no difference in disease severity between the probiotics and no probiotics arms (p=0.32). Median time to disease resolution was 4 days. No difference in time to disease resolution was observed between patients never on probiotics and patients on established probiotic regimens (p=0.64). There was a significant increase in time to resolution in patients starting probiotics >24 hours after CDI diagnosis (p=0.03).
Conclusion: Probiotics increase pill burden as well as cost to patients and healthcare systems, without ameliorating disease severity or time to disease resolution.
Background
Clostridioides difficile (C. difficile) is the most common cause of hospital-related diarrhea and the most common nosocomial infection [1]. C. difficile is a Gram-positive, spore-forming, anaerobic intestinal bacterium that is transmitted via the fecal-oral route. Upon exposure to bile acid, the spores germinate and subsequently can colonize an individual’s gastrointestinal tract. In an otherwise healthy host, normal intestinal flora prevent C. difficile overgrowth and infection. However, in persons with risk factors, most notably recent exposure to antibiotics, C. difficile can lead to infection characterized by profuse watery diarrhea [2].
Initial episode of C. difficile infection (CDI) is classified by the Infectious Disease Society of America (IDSA) Guidelines as non-severe, severe, or fulminant based on specified clinical data. Severe CDI is diagnosed based on a white blood cell count ≥ 15 000 cells/mL or a serum creatinine level > 1.5 mg/dL [1]. Although the most updated IDSA guidelines on the management of C. difficile recommend similar treatment courses for non-severe and severe CDI, studies have shown a significant difference in outcomes, prognosis, and burden on the healthcare system based on disease severity stratification. Patients with severe CDI have prolonged hospitalizations, increased need of intensive care management, increased risk of colectomy, and increased risk of mortality [3-5].
There is currently no consensus on the role of probiotics in CDI therapy, and specifically, in severe CDI [1,6,7]. While multiple beneficial roles of probiotics have been proposed [8], the data from clinical studies are conflicting [9-12]. In-vitro and pre-clinical studies have shown that probiotics can replenish the normal gut flora in patients who received antibiotics, provide intestinal barrier protection, modulate the innate and adaptive immune system and increase phagocytosis, and provide inherent antimicrobial activity [8,11]. Multiple studies have looked at the effects on incidence of first CDI occurrences [9,13] and recurrent CDI [14,15], with varying results between studies, yet these authors have been unable to find any studies that examined the effects of probiotics on incidence of severe CDI versus non-severe CDI, nor studies examining the effects of probiotics on time to disease resolution (soft or formed stool).
The purpose of this study is to explore a new place in therapy for probiotics in the setting of CDI. We aim to compare the incidence of severe CDI in patients taking probiotics versus no probiotics and to establish any differences in time to CDI resolution.
Methods
CDI case obtainment
This was a retrospective, single-center, cohort study of CDI positive cases at NYU-Winthrop Hospital from August 2015 through February 2017. This study was granted exempt status by the NYU-Winthrop’s institutional review board. A list of CDI-positive cases during our study timeframe was collected from the hospital’s microbiology lab. Patients were included if they were at least 18 years of age and had a positive C. difficile toxin B (tcdB) gene detected by PCR. Patients were excluded if they were missing CDI severity lab markers, when the available markers indicated non-severe CDI, and if the time to soft or formed stool was not documented. The electronic medical record (EMR) was used to collect the following data points: age, gender, use of antibiotics, use of probiotics, probiotic strains used, markers of CDI severity, number of loose stools at time 0 and 72 hours, time to soft or formed stool, and CDI course of treatment.
CDI diagnosis
Stools samples were sent to the microbiology lab for C. difficile testing in patients who presented with watery stools. CDI diagnosis was made via rapid detection of tcdB by real-time PCR.
Defining study outcomes
CDI severity was defined based on IDSA guidelines. Patients were determined to have severe CDI if they had any one of the following: white blood cell count > 15,000 cells/µL, serum creatinine > 1.5 mg/dL, or hypotension [1]. Hypotension was not clearly defined by the IDSA guidelines, so we used the criteria provided by the National Heart, Lung, and Blood Institute of systolic blood pressure < 90 mmHg or diastolic blood pressure < 60mmHg [19]. Albumin levels were collected, as well. Although albumin is not included in the IDSA’s CDI severity criteria, historically, many papers have used hypoalbuminemia as a marker of severe CDI [3,5,20].
Time to CDI resolution was defined as time to non-watery stools. The EMR progress notes were searched for stools described as “soft,” “formed,” or “non-watery.” We also determined CDI to be resolved if a patient had no stools in a 24-hour period.
Probiotic strains
At NYU-Winthrop Hospital, providers have the option of two probiotics. Lactobacillus acidophilus is on formulary. A serving size of 2 caplets provides 50 million colony forming units. Sacchromyces boulardii is available as a non-formulary alternative.
Distinguishing probiotic use groups
We had two probiotic use groups in our incidence of severe CDI analysis- no probiotics and probiotics.
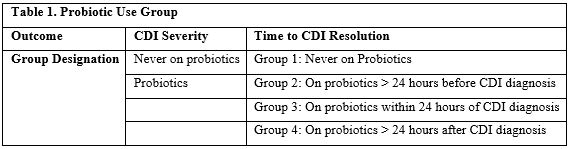
However, at the onset of data collection, we found that those two groups did not accurately represent the probiotic treatment course of our patients when analyzing time to disease resolution. To better portray the effects of probiotics on time to CDI resolution, we divided our patients into four probiotic use groups as follows (Table 1): never on probiotics (group 1), probiotics started at least 24 hours prior to CDI diagnosis (group 2), probiotics started within 24 hours of CDI diagnosis (group 3), and probiotics started at least 24 hours after CDI diagnosis (group 4).
Statistical Analysis
Descriptive statistics (mean, standard deviation, median, 25th and 75th percentiles, minimum and maximum values for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group. The chi-square test or Fisher’s exact test, as deemed appropriate, was used to compare the groups for categorical variables. For the two group comparisons, the two-sample t-test or Mann-Whitney test was used for continuous data. For four group comparisons, analysis of variance (ANOVA) or the Kruskal-Wallis test was used.
The analysis of “Time to Disease Resolution” was accomplished by applying standard methods of survival analysis, i.e., computing the Kaplan-Meier[1] product limit curves, where the data was stratified by group. In cases where the endpoint event had not yet occurred, the time until last follow-up was used and considered ‘censored’. The groups were compared using the log-rank test.
A result was considered statistically significant at the p<0.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Table 1: Probiotic Use Groups.
Results
Two hundred and ten cases of tcdB gene were detected from August 2015 through February 2017.
Disease Severity
All 210 cases were included in the CDI disease severity analysis (Figure 1). There were 169 patients in the no probiotics arm and 41 patients in the probiotics arm. There were no significant baseline characteristic differences between the two groups (Table 2). The median age was 75 years (range 18.6-97.5 years) and 57% of patients were female. Over 80% of patients were on antibiotics, and presumably more had taken antibiotics within 12 weeks of their diagnosis. Of the patients who were taking probiotics, over 97% were taking L. acidophilus and 2.4% were taking S. boulardii.
Sixty-six percent of patients never on probiotics presented with severe CDI compared to 58% of patients in the probiotics group (p=0.32) (Figure 2). We found no significant differences between the groups in any one of the markers for severe CDI, including white blood cell count, serum creatinine, blood pressure, and albumin.
Time to Disease Resolution
One hundred and ninety cases were included in the time to CDI resolution analysis. Cases were distributed in the following way: 86 patients in group 1, 39 patients in group 2, 41 patients in group 3, and 24 in group 4 (Figure 1). There were no significant differences in baseline characteristics among the 4 groups (Table 3). Median age was 74.4 years (range 18.6-97.5 years) and 55.3% of patients were female. Eighty-one percent of patients were on antibiotics and, similar to the disease severity group, presumably more had taken antibiotics within 12 weeks of their diagnosis. Of the patients taking antibiotics, 96% were taking L. acidophilus. We also looked for differences in CDI treatment regimens as this could have been a confounding factor in the time to CDI resolution analysis. There was no significant difference among the groups in the use of oral vancomycin, metronidazole, and tigecycline.
The median time to soft or formed stool was 4 days (range 1-18 days). In group 1 (patients who were never on probiotics), group 2 (patients who were on probiotics at least 24 hours prior to CDI diagnosis), group 3 (patients who were started on probiotics within 24 hours of CDI diagnosis), and group 4 (patients who were started on probiotics greater than 24 hours after CDI diagnosis), the median time to soft or formed stools was 4 days, 3 days, 4 days, and 6 days, respectively. There was a significant difference in time to CDI resolution among the four groups (p < 0.034) (Figure 3a). In group 4, the group that was started on antibiotics at least 24 hours after diagnosis, the median time when patients were initiated on probiotics was 3 days after CDI diagnosis. Therefore, we do not attribute the difference in time to resolution to the use of probiotics, rather these patients had a prolonged recovery from CDI and probiotics were added in desperation when patients did not show response to traditional antibiotic therapy.
We analyzed our dataset in different ways to further explore if probiotics made a significant difference in CDI resolution. When we compared group 1 (patients never on probiotics) to group 2 (patients on probiotics for at least 24 hours prior to CDI diagnosis), the median time to disease resolution was 4 days and 3 days, respectively (p < 0.63) (Figure 3b). We then combined group 1, group 3, and group 4 together, representing a cohort who had not been on an established probiotic regiment prior to developing CDI. The median time to CDI resolution for these groups when analyzed together was 4 days. Once again, there was no significant difference when compared to group 2 (on probiotics at least 24 hours prior to CDI diagnosis) (p < 0.34) (Figure 3c).
Table 2: Characteristics of Patients Included in CDI Severity Analysis.

Table 3: Characteristics of Patients Included in Time to CDI Resolution Analysis.
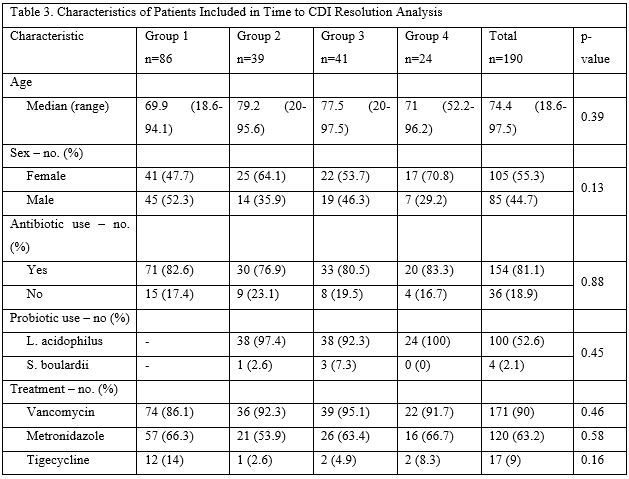

Figure 1: Study Flowchart.
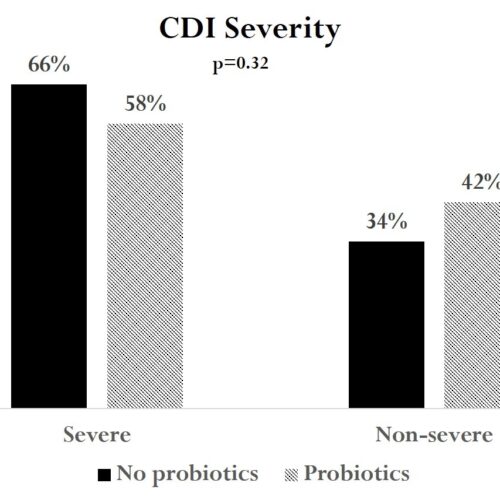
Figure 2: Rates of Severe and Non-severe CDI in Patients on No Probiotics versus Probiotics.
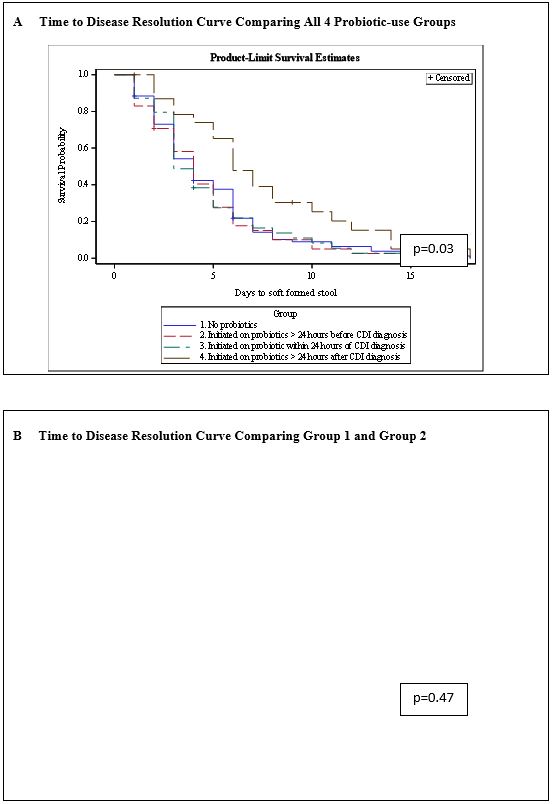
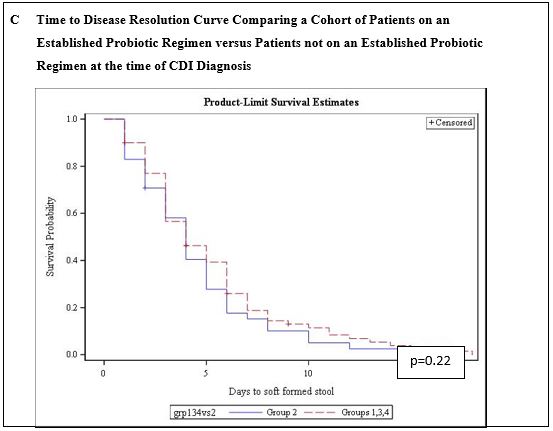
Figure 3: Kaplan-Meier Curves Representing Time to CDI Resolution and Comparing Probiotic-use Groups in Multiple Analyses.
Discussion
The role of probiotics in the prevention and management of C. difficile is not clearly defined from the perspective of any clinical guidelines [1, 6-7]. The 2013 American College of Gastroenterology Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections state that there is insufficient evidence that probiotics prevent CDI and that there is limited evidence for the use of adjunct probiotics to decrease recurrent CDI [7]. A 2015 Delphi panel published its guidelines on preventing nosocomial CDI. They provide no recommendation for adjunct probiotics to prevent recurrence [6]. The Infectious Disease Society of America’s 2017 Clinical Guidelines for Clostridium difficile Infection similarly state that there is insufficient data to recommend administration of probiotics for primary prevention of CDI [1]. These guidelines also advocate for more research to be conducted.
The above guidelines focus on the role of probiotics in preventing first occurrences of CDI and recurrences because that is what most of the primary literature has looked at. However, there are other aspects of CDI management that could benefit from adjunctive therapy. For example, decreasing the incidence of severe CDI could mean less ICU requirements, shortened hospitalization courses, and decreased medical costs. Shortening the time to CDI resolution would also improve patients’ quality of life and decrease costs. Thus far, these authors have been unable to find primary literature that evaluated the use of probiotics for these endpoints. In 2014, Bakken published his results on using a treatment regimen of staggered and tapered antibiotics withdrawal plus oral liquid Lifeway kefir in place of fecal microbiota transplantation for recurrent CDI [16]. He found that 80% of patients remained diarrhea-free for at least 12 months. Spinler et al. administered this same kefir to a CDI mouse model in an effort to explore the mechanisms of its protective effect.17 Spinler unexpectedly found that the kefir drastically increased disease severity, with all of the mice receiving Lifeway kefir having more weight loss and quicker health decline than the comparator group.
There are studies showing benefits of probiotics in the management of CDI, but we must regard these results with reservations. One study was biased by its failure to control for type, duration, and dose of antibiotics and another study only found a benefit in a post-hoc analysis of only patients on high doses of oral vancomycin [12], a CDI treatment strategy that has seen been proven to be superior to conventional oral vancomycin dosing [18]. Furthermore, some studies that showed benefits with probiotic use, used specific strands of probiotics. In fact, the Delphi panel recommended using Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC0R to prevent CDI [6]. This highlights the difficulty of performing research with probiotics. Unlike FDA-approved medications, probiotics are not standardized and there are dozens of single-strains and combinations of strains of probiotics that can be used.
This study adds to the growing body of literature recommending against the use of probiotics in the setting of CDI and this is the first study to assess the risk of severe CDI in patients on probiotics versus no probiotics.
There are some limitations to our study. First, this was a non-randomized, retrospective chart review. We were reliant on stool documentation and some subjects required censoring if stools were not soft or formed by the time of discharge. Second, our results cannot be generalized to all probiotic strains and doses. However, we used a reputable and popular strain at the recommended dosage range. Finally, CDI was diagnosed via real-time PCR, as opposed to a multistep algorithm proposed by IDSA. This could potentially result in an over-diagnosis of CDI, but our 3-4 day median time to soft stool leads us to believe our cases were true CDI.
Conclusion
This study found that probiotics had no utility in ameliorating the incidence of severe CDI. Furthermore, probiotics made no impact in shortening the time to CDI resolution. Patients who were started on probiotics at least 24 hours after CDI diagnosis had a significantly longer time to CDI resolution than the other groups. But they were started on probiotics around the time when other groups were experiencing CDI resolution. Like previous studies that saw no benefit in the use of probiotics to prevent CDI, we saw no benefit in preventing severe CDI or shortening time to disease resolution. We recommend against the use of probiotics in this setting as probiotics ultimately increase pill burden and cost to patients without added benefits of mitigating CDI.
References
- McDonald L, Gerding D, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clinical Infectious Diseases. 2018; 66(7): 987-994. doi:10.1093/cid/ciy149.
- Seekatz A, Young V. Clostridium difficile and the microbiota. Journal of Clinical Investigation. 2014; 124(10): 4182-4189. doi:10.1172/jci72336.
- Lungulescu O, Cao W, Gatskevich E, Tlhabano L, Stratidis J. CSI: a severity index for Clostridium difficile infection at the time of admission. Journal of Hospital Infection. 2011; 79(2): 151-154. doi:10.1016/j.jhin.2011.04.017.
- Na X, Martin A, Sethi S, et al. A Multi-Center Prospective Derivation and Validation of a Clinical Prediction Tool for Severe Clostridium difficile Infection. PLoS ONE. 2015; 10(4): e0123405. doi:10.1371/journal.pone.0123405.
- Miller M, Louie T, Mullane K, et al. Derivation and validation of a simple clinical bedside score (ATLAS) for Clostridium difficile infection which predicts response to therapy. BMC Infect Dis. 2013; 13(1). doi:10.1186/1471-2334-13-148.
- Goldstein E, Johnson S, Maziade P, et al. Pathway to Prevention of Nosocomial Clostridium difficile Infection. Clinical Infectious Diseases. 2015; 60(suppl 2): S148-S158. doi:10.1093/cid/civ142.
- Surawicz C, Brandt L, Binion D, et al. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. American Journal of Gastroenterology. 2013; 108(4): 478-498. doi:10.1038/ajg.2013.4.
- Ollech J, Shen N, Crawford C, Ringel Y. Use of probiotics in prevention and treatment of patients with Clostridium difficile infection. Best Practice & Research Clinical Gastroenterology. 2016; 30(1): 111-118. doi:10.1016/j.bpg.2016.01.002.
- Hickson M, D'Souza A, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised doubleblind placebo-controlled trial. BMJ. 2007; 335(7610): 80. doi:10.1136/bmj.39231.599815.55.
- Goldenberg J, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database of Systematic Reviews. 2017. doi:10.1002/14651858.cd006095.pub4.
- Parkes G, Sanderson J, Whelan K. The mechanisms and efficacy of probiotics in the prevention of Clostridium difficile-associated diarrhoea. The Lancet Infectious Diseases. 2009; 9(4): 237-244. doi:10.1016/s1473-3099(09)70059-3.
- Pillai A, Nelson R. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database of Systematic Reviews. 2008. doi:10.1002/14651858.cd004611.pub2.
- Allen S, Wareham K, Wang D, et al. A high-dose preparation of lactobacilli and bifidobacteria in the prevention of antibiotic-associated and Clostridium difficile diarrhoea in older people admitted to hospital: a multicentre, randomised, double-blind, placebo-controlled, parallel arm trial (PLACIDE). Health Technol Assess (Rockv). 2013;17(57). doi:10.3310/hta17570.
- Surawicz C, McFarland L, Greenberg R, et al. The Search for a Better Treatment for Recurrent Clostridium difficile Disease: Use of High‐Dose Vancomycin Combined with Saccharomyces boulardii. Clinical Infectious Diseases. 2000; 31(4): 1012-1017. doi:10.1086/318130.
- Wullt M, Hagslätt M, Odenholt I. Lactobacillus plantarum 299v for the Treatment of Recurrent Clostridium difficile-associated Diarrhoea: A Double-blind, Placebo-controlled Trial. Scand J Infect Dis. 2003; 35(6-7): 365-367. doi:10.1080/00365540310010985.
- Bakken J. Staggered and Tapered Antibiotic Withdrawal with Administration of Kefir for Recurrent Clostridium difficile Infection. Clinical Infectious Diseases. 2014; 59(6): 858-861. doi:10.1093/cid/ciu429.
- Spinler J, Brown A, Ross C, Boonma P, Conner M, Savidge T. Administration of probiotic kefir to mice with Clostridium difficile infection exacerbates disease. Anaerobe. 2016; 40: 54-57. doi:10.1016/j.anaerobe.2016.05.008.
- Cunha BA, Sessa J, Blum S. Enhanced efficacy of high dose oral vancomycin therapy in clostridium difficile diarrhea for hospitalized adults not responsive to conventional oral vancomycin therapy: antibiotic stewardship implications. J Clin Med. 2018; 7: e75. doi: 10.3390/jcm7040075.
- Low Blood Pressure. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/low-blood-pressure. 2019.
- Zar F, Bakkanagari S, Moorthi K, Davis M. A Comparison of Vancomycin and Metronidazole for the Treatment of Clostridium difficile-Associated Diarrhea, Stratified by Disease Severity. Clinical Infectious Diseases. 2007; 45(3): 302-307. doi:10.1086/519265.

