Use of Anticholinergic Drugs in People Referred to an Urban Memory Clinic Service Setting
Ina Sawhney1,*, Angela Taylor1, Soghra Ather1, Elizabeta B Mukaetova-Ladinska1,2
1The Evington Centre, Leicestershire Partnership NHS Trust, UK
2Department of Neuroscience, Psychology and Behaviour, University of Leicester, UK
Received Date: 08/08/2021; Published Date: 31/08/2021
*Corresponding author: Ina Sawhney, MRCPsych, The Evington Centre, Leicester General Hospital, Gwendolen Road, Leicester LE5 4QG, UK
Abstract
Introduction: Drugs with anticholinergic properties have a series of adverse effects among older people, including memory problems, delirium, falls and fractures, and their use may outweigh health benefits. Reducing or stopping the anticholinergic medication can contribute to improving both their cognitive/mental and physical health.
Method: The Audit sample of 316 patients was selected retrospectively from all new referrals to the Memory Service at Leicestershire Partnership NHS Trust over a period of 6 months (July and December 2018; n=1,400), selecting every fourth patient on the list of referrals generated. Using the Anticholinergic Cognitive Burden scale, we analysed the use of anticholinergic drugs recorded as per GP letters.
Results: 57.28% (n=181) of list of medication included any anticholinergic medication contained in the Anticholinergic Cognitive Burden (ACB) Scale, with only one clinician having documented the potential effect on cognition of the anticholinergic medication(s). The most frequently prescribed anticholinergic drugs were antidepressants (28.44%) and opioids (18.44%).
Conclusions: The high rate of anticholinergic prescribing among older people calls for revision of the current prescribing policies to optimise the older people’s health. In particular the use of opioids and antidepressants needs to be revised regularly to minimise the anticholinergic burden.
Keywords: Memory clinic; Older people; Anticholinergic medication; Primary care; Dementia
Introduction
Dementia is a leading cause of disability and death, and its prevention is a global public health priority. Dementia is caused by a number of different neurodegenerative processes that contribute to irreversible cognitive decline and associated symptoms, such as the progressive loss of independence and daily functioning. No disease modifying treatments for dementia exist; however, age specific dementia incidence across populations is declining [1], suggesting that changing lifestyles (i.e. diet, physical activity and cognitive engagement; [2]) or environment (i.e. air pollution, lack of vitamin D; occupational exposure to some types of pesticide; [3,4]) may lead to a meaningful change in the prevalence of dementia risks. Indeed, these modifiable dementia risk factors contribute towards 40% of the risk for developing dementia [5]. Hence identifying and reducing the exposure to risk factors that can affect any aspect of long-term brain health is important for dementia prevention and cognitive health in the population.
Drug consumption in elderly people is high, and many commonly prescribed drugs have anticholinergic/antimuscarinic effects (such as antiemetics, antispasmodics, bronchodilators, antiarrhythmic drugs, antihistamines, analgesics, antihypertensives, antiparkinsonian agents, corticosteroids, skeletal muscle relaxants, ulcer drugs, and psychotropic drugs) [6]. Furthermore, such drugs are likely to have a more toxic effect in an ageing brain because of increased permeability of the blood brain barrier, slower metabolism and drug elimination, and polypharmacy [7] and result in reduced total cortical volume and temporal lobe cortical thickness and greater lateral ventricle and inferior lateral ventricle volumes [8]. Some commonly prescribed medicines are associated with increased anticholinergic burden, and therefore interfere with cognitive impairment and are associated with increased risk for dementia [9], frailty [10] and high mortality rates [11]. Minimising the use of these medicines and if possible looking for alternatives should be considered.
Method
In order to address the anticholinergic prescribing and minimise their use in the older people referred to the Leicestershire Partnership NHS Trust (LPT), we conducted an Audit. The Audit sample was based on 316 patients selected retrospectively from all new referrals to the Memory Service at Leicestershire Partnership NHS Trust over a period of 6 months (from 1st July until 31st December 2018). For this, every fourth patient on the list of 1,400 referrals generated was selected. Majority of the participants were white (n=265), with only 16.14% of participants (n=51) from ethnic minorities, predominantly South Asians (n=48), and 3 people from Caribbean African ethnic background.
The use of anticholinergic drugs (using the Anticholinergic Cognitive Burden scale; http.acbsalc.com) as per General Practitioners’ (GP) letters was recorded independently by three clinicians, all specialists in old age psychiatry (IS, SA, AT). The ACB scale assigns anticholinergic activity based on receptor binding studies and report clinically relevant anticholinergic adverse events, thus showing much higher anticholinergic burden than other scales used for anticholinergic burden estimates, such as the Anticholinergic Risk Scale (ARS), and the Drug Burden Index anticholinergic subscale (DBI-ACH; [12]). The ACB score was calculated taking into account drugs that were on repeated prescription (>3 months’ duration), whereas drugs that were prescribed acutely were not taken into account.
Statistical Analysis
A database, containing demographic information about gender, age at the time of referral and ethnicity, alongside comorbidities and use of anticholinergic medication was created with Microsoft Excel and used to to determine ACB frequencies and percentages of use for separate anticholinergic drugs. The audit standard was determined according to the NICE guidance for dementia 2018 [13] (Table 1).
Table 1: Audit standards for ACB prescribing. The audit standards determined after the NICE guidance for dementia (2018) [13].

Results
Anticholinergic drugs prescribing
The list of medication was included in 97% of the GP referral letter. Anticholinergic medication, contained in the Anticholinergic Cognitive Burden scale (ACB), as per the GP referral letters, featured frequently in the regular repetitive medication list. Thus, more than half of people referred to our memory clinic were on at least one anticholinergic medication (n= 181; 57.28%). Majority of them (56.91%) were using anticholinergic drugs with low anticholinergic potency (ACB scores 1and 2, 41.99 and 14.92%, respectively) (Figure 1). However, 78 (43.09%) had a regular medication of anticholinergic drugs of an ACB score of ≥3 (ACB score 3-9), indicating higher risk of confusion, falls and death.
Most commonly prescribed anticholinergic drugs
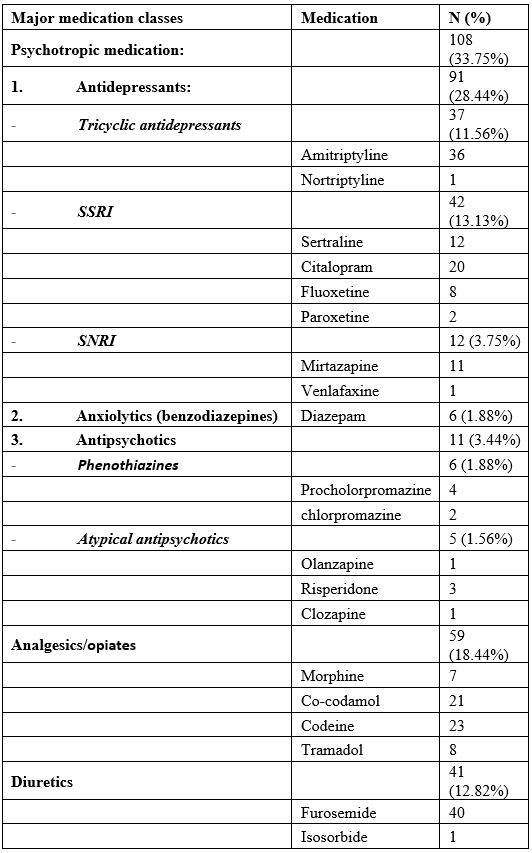
The 181 patients on anticholinergic drugs had a total of 320 prescription for 37 anticholinergic drugs: antidepressants, (SSRI, SNRIs, and tricyclic antidepressants; n=91; 28.44%), heart failure (digoxin, warfarin, also including diuretics, i.e. furosemide; n=36 and n=40, respectively; 23.75%), pain control (morphine, co-codamol, codeine and tramadol; n=59, 18.44%); antiallergics (ceterizine and larotidine, n=17; 5.31%), urinary incontinence (oxybutynin, solifenacin, tolterodine; n=15, 4.69%), H2 blockers (i.e ranitidine, n=12; 3.75%) and antiparkinsonian medication (sinemet, co-levodopa; n=7, 2.19%) (Table 2). Only 5 people referred to the MHSOP were prescribed neuroleptic medication; one each olanzapine and clozapine, as a maintenance therapy for a chronic psychotic illness (schizophrenia), whereas three were on risperidone, due to behavioural and psychological symptoms of dementia, i.e. hallucinatory and delusional believes as a result of memory problems. Benzodiazepines were not used frequently, with only 6 people (3.35%) using diazepam regularly.
Table 2. Most frequently used anticholinergic medications. 65% of prescribed anticholinergic drugs were due to psychotropic medication (33.75%), analgesics and opiates (18.44%) and diuretics (12.82%).
Are anticholinergic drugs reviewed?
Although this high anticholinergic burden warrants a review of the medication (i.e. considering switching them to a lower-risk alternative), and also discussion with both patients and relatives/carer (https://www.prescqipp.info/our-resources/bulletins/bulletin-253-anticholinergic-burden/), this was not considered routinely, with only one clinician documenting the potential effect on cognition of the anticholinergic medication(s), following a recommendation by a consultant physician about solifenacin (which is on the ACB list) to be stopped due to concern it may be affecting cognition. Similarly, only one clinician informed the GP of their potential effect on cognition, and also asked them to consider a suitable alternative or stop the anticholinergic medication(s). Only 2 clinicians documented to have discussed the anticholinergic medication(s) with their patient/ carer.
Of interest to note there were 21 patients (6.65%) who had previous adverse reaction to a total of 32 anticholinergic medication (1-13 years prior current referral to MHSOP, predominantly to opioids (co-codamol, codein, dihidrocodeine and morphine 5:2:1:1) and SSRIs (i.e. paroxetine, sertraline and citalopram, 1:3:3) and amitriptyline (n=2), and it has been discontinued. Only four of them were later prescribed anticholinergic medication and had no adverse effects.
Discussion
Our study confirmed routine use of anticholinergic drugs in more than half of older people referred over a six months period to a memory clinic. Our reported higher rate of prescribing anticholinergic medication is in sharp contrast to the observed decreasing trend during the last decade [14], the implementation of the UK national regulative, i.e. the NICE guidance on multimorbidity and polypharmacy in 2017 [15], the NICE guidance for dementia (2018) [13], as well as the 2016 Health Survey England [16], that documented widespread polypharmacy among the older UK people. Bearing in mind that our audit was conducted shortly after the above recommendations were put in place, it is not surprising that they had very limited influence upon the prescribing culture among primary and secondary/tertiary care physicians in our region, and thus have not contributed to the recommended reduction of inappropriate prescribing of anticholinergic/antimuscarinic drugs in community dwelling older people [17]. Future studies may shed further light whether the above recommendations have been both accepted by medical professionals and contribute to raising awareness of the anticholinergic side effects among service user, and determine their impact on the quality of healthcare and outcomes for older people.
Another reason for the high percentage of ACB drug use in the current study may be due to the choice of the ACB assessment tool. Thus, an earlier (paper) version of the ACB tool (2008) did not include a number of anticholinergic drugs (i.e. diazepam, and the full list of SSRIs and SNRIs antidepressants, with only paroxetine and mirtazapine included), whereas fluoxetine and diazepam were added in the 2012 ACB version. The latest web ACB tool, however, contains all 4 SSRIs and 2 SNRIs (mirtazapine and venlafaxine) antidepressants, but not duloxetine. When assessing the ACB score on this analysed cohort, using the 2012 paper version of the tool, we detected only 55 people were prescribed ACB drugs bringing down the rate of ACB prescribing to 17.41%. Thus, only two people were identified to have been using diazepam regularly, and additional two SSRI and SNRI (paroxetine and venlafaxine) antidepressants, in contrast to the current revised ACB study in which 52 additional SSRI (n=41) and SNRI (n=11) antidepressant users were identified. When using the 2008 ACB version, the frequency of the prescribed anticholinergic drugs was, not surprisingly, significantly lower and similar to that reported to the prescribing rate of potential or definite anticholinergic properties (ACB>0) to that described for Italian memory clinic users (15.9%; [18]), whereas the latest revision of 57.2% is similar to the ACB prescribing reported in community-dwelling Australian people who attended 9 memory clinics (59.69%; [19]) and had a diagnosis of mild cognitive impairment or dementia [11].
Not surprisingly, the most prescribed anticholinergic drugs were those who are used for treatment of pain (i.e. amitriptyline and codein /co-codamol/morphine/tramadol), antidepressants (SSRI and SNRI), heart failure and fluid retention (digoxin/warfarin/furosemide). Furthermore, most of these drugs have been used over a prolong period of time (i.e. amitriptyline, atenolol, diazepam), and have either not been substituted over the years, or substituted with an alternative with similar anticholinergic properties. The higher rate of ACB prescribing among the above conditions has been further confirmed in our secondary analysis, where hypertension, diabetes, cardiovascular, renal and respiratory conditions were linked to significant increase in the ACB prescribing, i.e. up to 1.4-2.3 fold higher, whereas the group of mixed medical conditions i.e. gastrointestinal problems, skin conditions (i.e. psoriasis, pruritus, vitiligo etc.), central nervous system conditions (i.e. epilepsy, polyneuropathy, multiple sclerosis, Bell’s paralysis etc.), was the medical group were ACB drugs were not commonly prescribed (EBM-L et al, unpublished). However, in contrast to a previous study, in which most common anticholinergic classes used were tricyclic antidepressants, first-generation antihistamines, and bladder antimuscarinics [20], this was not the case in our study. Although the number of people who were on antimuscarinic medication for overactive bladder was relatively low, we noticed a differential mode of prescribing, similar to that described in a larger population-based study, with slight preference of solfenicine over oxybutynin [21].
Similarly, to a previous study [14] where the prescription of antidepressants was double the other ACB drugs, we found high rate of prescription of antidepressants, with amitriptyline relatively more frequently prescribed and used largely for neuropathic pain control and insomnia (as based on the prescribed dosage of 10-20mg daily) rather as an antidepressant. However, the use of newer antidepressants (e.g. SSRIs and SNRIs) was more common (nearly in 17% of our sample), with no clinical evidence for treatment of enduring mental health problems, i.e. depression and/or anxiety. The relatively high rate of antidepressant prescribing is in accord with the latest reports of estimated 5-16% of adults receiving antidepressants in Europe and the USA annually [22]. The reason for the increased prescribing of antidepressants in community dwelling older people may be attributable to primary care physicians seeing people in distress and crises, and also being confident in using the antidepressants beneficial properties (i.e. sedative effects helping insomnia and anxiety symptoms and gain weight) [23]. In addition, the sedative properties of the low dose antidepressants (i.e mirtazapine) seems to have been adopted as a safer non-addictive alternative to avoid benzodiazepines [23], as documented via the sparse use of benzodiazepines in our older people referred to out memory clinic. Namely only 6 people were prescribed diazepam (1.88%).
Shortly after completing the audit, in November 2019 the Medicines & Healthcare products Regulatory Agency (MHRA, responsible for regulating all medicines and medical devices in the UK by ensuring they work and are acceptably safe) issued an alert to healthcare professionals, recalling all unexpired stock of certain batches of prescription-only Ranitidine medicines used to treat conditions such as heartburn and stomach ulcers. This was a precautionary measure due to possible contamination with NDMA (N-nitrosodimethylamine), which has been identified as a risk factor in the development of certain cancers. Nevertheless, the ranitidine discontinuation should not affect overtly the extent of the ACB prescribing in older people, as based on our study, since only 12 of our patients had been issued with this medication, representing 3.75% of all the ACB prescription.
Chronic pain is rather common among older people, with the opioid-based pain management being much more prevalent in this age group (reviewed in [24]). It is, thus, not surprising that opioids featured among the most frequently prescribed anticholinergic drugs (18.44%). However, our study was limited to patients’ repeated medication charts from primary care and did not include opioids available over the counter, and, thus, may be an underestimate of the use of these drugs. Additional 11.25% patients used low doses amitriptyline for musculoskeletal and chronic non-cancer pain, despite the recommendations to avoid tertiary tricyclics such as amitriptyline due to concerns over adverse side effects. Although the recommendations are to consider nortriptyline instead of amitriptyline [25], in our study, only one patient was prescribed nortriptyline.
Polypharmacy is the major risk factor for adverse side effects in older people (i.e. nausea light-headedness, muscle weakness and pain, bleeds, confusion, falls, sedation, altered clothing capacity etc.), which can lead to serious, but preventable injuries and illnesses [6]. Furthermore, the cumulative exposure (combined score) to anticholinergic and sedative drugs is significantly associated with an increased risk for frailty [95%CI OR 3.54 (1.47-8.57)] [10]. One of the interventions aimed at reducing this risk is the detection and reduction of inappropriate and harmful prescriptions. The responsibility of the drug reviews, thus, lies upon all clinicians involved in the medical care of older people. In memory clinical setting, we propose regular addressing of risks/benefits of the anticholinergic medication by both independent clinician reviews, as well as at multidisciplinary team meetings and actioned accordingly. Risks of anticholinergic cognitive burden should be highlighted in the letter to GP. If deemed appropriate, GP should be asked to review the medication in the care plan, actions that have been significantly missing in the referrals to secondary/tertiary care.
Though comorbidities proportionally increase with ageing, regular review of repeat drug prescriptions, especially those with known anticholinergic burden needs to be incorporated regularly in routine clinical setting. In addition, we propose immediate action on long standing use of ACB drugs especially opioids and tricyclic antidepressant and when only one of these ACB drugs is used. Regular pharmacological reviews will decrease the anticholinergic burden, improve the lives of older people, and also decrease their potential impact on their cognitive functioning. However, although opportunities for quality improvement of anticholinergic/muscarinic prescribing in older adults remain and they need to be explored and addressed individually for each patient, these may well be hampered by cost effectiveness and social value judgements.
Acknowledgement: None.
Competing interests: None.
Ethics approval statement: The study was approved by the LPT Medicines Audit Group,
and discussed at the clinical network.
Contributorship statement: Dr. Sawhney provided the leadership and idea for the research.
Drs Sawhney,Taylor and Ather collected the data and created the database. Dr. Sawhney and
Prof. Mukaetova completed the analysis of data and wrote the paper, with all authors
contributing during its finalisation. All authors have approved the final version of the
manuscript.
References
- Wolters FJ, Chibnik LB, Waziry R, Anderson R, Berr C, Beiser A, et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology 2020; 95(5): e519-e531. doi: 10.1212/WNL.0000000000010022.
- McMaster M, Kim S, Clare L, Torres SJ, Cherbuin N, DʼEste C, et al. Lifestyle risk factors and cognitive outcomes from the multidomain dementia risk reduction randomized controlled trial, Body Brain Life for Cognitive Decline (BBL-CD). J Am Geriatr Soc doi: 10.1111/jgs.16762.
- Killin LO, Starr JM, Shiue IJ, Russ TC.Environmental risk factors for dementia: a systematic review. BMC Geriatr 2016; 16(1): 175. doi: 10.1186/s12877-016-0342-y.
- Thomson EM.Air Pollution, stress, and allostatic load: Linking systemic and Central Nervous System impacts. J Alzheimers Dis 2019; 69(3): 597-614. doi: 10.3233/JAD-190015.
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. 2020; 396(10248): 413-446. doi: 10.1016/S0140-6736(20)30367-6.
- Cantlay A, Glyn T, Barton N. Polypharmacy in the elderly. InnovAiT 2016; 9(2): 69–77.
- Malaz Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: A review and practical application. Aging Health 2008; 4(3): 311-320. doi: 2217/1745509X.4.3.311.
- Risacher SL, McDonald BC, Tallman EF, West JD, Farlow MR, Unverzagt FW, et al. Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73(6): 721-732.doi: 10.1001/jamaneurol.2016.0580.
- Coupland CAC, Hill T, Dening T , Morriss R, Moore M , Hippisley-Cox Anticholinergic drug exposure and the risk of dementia: A nested case-control study. JAMA Intern Med 2019; 179(8): 1084-1093. doi: 10.1001/jamainternmed.2019.0677.
- Reallon E, Chavent B, Gervais F, Dauphinot V, Vernaudon J, Krolak-Salmon P, et al. Medication exposure and frailty in older community-dwelling patients: A cross-sectional study. Int J Clin Pharm 2020; 42(2): 508-514.doi: 10.1007/s11096-020-01007-2.
- Cross AJ, George J, Woodward MC, Ames D, Brodaty H, Connors MH, et al. Potentially inappropriate medication, anticholinergic burden, and mortality in people attending Memory Clinics. J Alzheimers Dis 2017; 60(2): 349-358. doi: 10.3233/JAD-170265.
- Pont LG, Nieln JTH, McLachlan AJ, Gnjidic D, Chan L, Robert G Cumming RG, et al.. Measuring anticholinergic drug exposure in older community-dwelling Australian men: A comparison of four different measures. Br J Clin Pharmacol 2015; 80(5): 1169–1175. doi: 1111/bcp.12670
- NICE Guidelines NG97. Dementia: Assessment, management and support for people living with dementia and their carers. Updated June 2018.
- Rhee TG, Choi YC, Ouellet GM, Ross JS. Rhee TG, et al.National prescribing trends for high-risk anticholinergic medications in older adults. J Am Geriatr Soc 2018; 66(7): 1382-1387. doi: 10.1111/jgs.15357.
- NICE KTT 18. Multimorbidity and Polypharmacy,
- Health Survey England, 2016.
- https://www.gov.uk/government/news/matt-hancock-orders-review-into-over-prescribing-in-the-nhs).
- Boccardi V, Baroni M, Paolacci L, Ercolani S, Longo A, Giordano M, et al. Anticholinergic burden and functional status in older people with cognitive impairment: Results from the Regal project. J Nutr Health Aging 2017; 21(4): 389-396. doi: 10.1007/s12603-016-0787-x.
- Cross AJ, George J, Woodward MC , Ames D, Brodaty H, Ilomäki J Elliott RA. Potentially inappropriate medications and anticholinergic burden in older people attending Memory Clinics in Australia. Drugs Aging 2016; 33(1): 37-44. doi: 10.1007/s40266-015-0332-3.
- Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al.Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175(3): 401-407. doi: 10.1001/jamainternmed.2014.7663.
- Vouri SM, Schootman M, Strope SA, Birge SJ, Olsen Differential prescribing of antimuscarinic agents in older adults with cognitive impairment. Drugs Aging 2018; 35(4): 321–331. doi: 10.1007/s40266-018-0531-9
- Prieto-Alhambra D, Petri H, Goldenberg JSB, Khong TP, Klungel OH, Robinson NJ, et al. Excess risk of hip fractures attributable to the use of antidepressants in five European countries and the USA. Osteoporosis Int 2014; 25(3): 847–855.
- Johnson CF, Williams B, MacGillivray SA, Dougall NJ, Maxwell 'Doing the right thing': factors influencing GP prescribing of antidepressants and prescribed doses.’ BMC Fam Pract 2017; 18(1): 72. doi: 10.1186/s12875-017-0643-z.
- Mikelyte R, Abrahamson V, Hill E, Wilson Factors influencing trends in opioid prescribing for older people: a scoping review. Prim Health Care Res Dev 2020; 21: e36. doi: 10.1017/S1463423620000365.
- Carrington Reid M, Eccleston C, Pillemer K. Management of chronic pain in older adults. BMJ 2015; 350: h532. doi: 10.1136/bmj.h532.

