Effects of Intravenous Lidocaine in Total Knee Joint Arthroplasty: A Randomized, Double-Blind, Placebo-Controlled Trial
Shun-Yu Wong*, David Ming-Hon Lam, Henry Chi-Yeung Mak, Chi-Wai Cheung, Timmy Chi-Wing Chan
Department of Anaesthesiology, Queen Mary Hospital, Hong Kong, China
Department of Anaesthesiology, University of Hong Kong, Hong Kong, China
Received Date: 11/07/2021; Published Date: 27/07/2021
*Corresponding author: Shun-Yu Wong, MBChB, Department of Anaesthesiology, Queen Mary Hospital, Hong Kong, China. Address: Room F2-43, 2nd Floor, Main Block, Queen Mary Hospital, 102 Pokfulam Road, Pokfulam, Hong Kong. Email: sywong.alex@gmail.com
Abstract
Background: We hypothesized that a single preemptive bolus dose of intravenous lidocaine (IV lidocine) would result in better analgesia, reduced total opioid consumption, shortened hospital length of stay (LOS) and better functional outcomes after unilateral primary Total Knee Arthroplasty (TKA).
Methods: 62 adults undergoing unilateral primary TKA were enrolled in this prospective, randomised, double-blind, placebo-controlled trial. They received either pre-incision IV lidocaine (2mg/kg) or placebo. All received standardized spinal anesthesia, local infiltration analgesia and postoperative analgesia. Primary endpoints were postoperative pain scores, measured by Numerical Rating Scale (NRS), and cumulative opioid consumption. Secondary outcomes included LOS, walking distance, active and passive range of movement (ROM), occurrence of Local Anesthetic Systemic Toxicity (LAST), constipation, nausea and vomiting (PONV), dizziness and Modified Barthel Index (MBI).
Results: Although not statistically significant, on both postoperative day 1 and 2, the lidocaine group demonstrated a lower NRS (at rest & upon movement) and longer walking distance averaged 5 metres more (p = 0.325, mean ± S.D. [95% C.I.] 61.00 ± 21.67m [53.09 - 68.91] vs. 55.00 ± 25.02 m [45.87 - 64.14]). Noticeably, the lidocaine group had significantly shorter LOS (p=0.0313, median [IQR] 3 days [3-4] vs. 4 days [3-4]). The 2 groups did not differ significantly regarding other secondary outcomes. Importantly, no LAST was recorded.
Conclusion: While a single pre-emptive bolus of IV lidocaine (2mg/kg) failed to attain statistically significant postoperative analgesia, this regime was safe and associated with shortened LOS by 1 day in patients undergoing unilateral TKA.
Trial registration: NCT0359776
Keywords: Intravenous lidocaine; Knee Arthroplasty; Acute pain; Anaesthesia, Spinal; Length of Stay
Introduction
Primary Total Knee Joint Arthroplasty (TKA) is among the most commonly performed procedures. Every year, while more than 2000 TKA are conducted in Hong Kong, over 10 000 patients are on the waiting list in the public sector [1].
TKA is associated with significant postoperative pain [2]. Poor pain control increases the risks of myocardial infarction, pneumonia and the development of chronic pain. It also impacts the recovery by delaying mobilization and prolonging the hospital stay [3].
Lidocaine is an amide-type local anaesthetic that has been repeatedly shown to be effective pain relief in major abdominal surgeries when administered as infusion perioperatively [4]. The mechanisms of action may involve the inhibition of N-methyl-d-aspartate (NMDA) receptors [5] and polymorphonnuculear (PMN) granulocyte priming [6]. Systemic lidocaine also inhibits the secretion of various inflammatory cytokines, such as IL-6, IL-8 and IL-1 Ra [7-8].
TKA involves substantial bone drilling and tissue injury, thus provoking a large inflammatory reaction. Similar to abdominal surgeries, systemic lidocaine may be effective analgesic and accelerate recovery in TKA. However, there is no randomized controlled trial done to study its effect on TKA.
We hypothesized that in adult patients undergoing TKA, a preemptive bolus dose of intravenous lidocaine would effectively reduce acute pain, decrease opioid consumption, improve rehabilitation and functional scorings in the initial postoperative period following TKA.
Methods
Study design
This was a prospective, single-center, double-blind, randomized, placebo-controlled trial. The trial was approved by the local university's Institutional Review Board (UW-18-26) and registered with an international clinical trials registry (www.clinicaltrials.gov, NCT0359776, https://clinicaltrials.gov/ct2/show/NCT03597776) before patient recruitment. Patients undergoing primary unilateral TKA were assessed for eligibility during preoperative screening visits. All eligible patients were informed about the study and written informed consent obtained. The study was conducted between January 2019 and January 2020 at the Duchess of Kent Children Hospital (DKCH), Hong Kong, in accordance with the ICH guidelines for Good Clinical Practice. CONSORT 2010 Statement was adopted as the reporting guideline. The full trial protocol is available to the reader upon request to the corresponding author.
Population
Eligible participants were all adults, aged 18-80 and with American Society of Anesthesiologists (ASA) physical status classification I-III, who were scheduled for primary unilateral TKA under spinal anaesthesia.
Exclusion criteria were defined as: any contraindications to or failed spinal anaesthesia, known intolerance or contraindication to local anaesthetics, paracetamol, non-steroidal anti-inflammatory drugs (NSAIDS) or opioids, single stage bilateral TKA / revision TKA, chronic pain other than knee pain, chronic use of opioids, substance abuse, cardiac disease (any degree of heart block / heart failure), any seizure disorder, psychiatric illness affecting pain perception; impaired renal function (defined as preoperative eGFR < 30ml /min /1.73 m2), impaired hepatic function, pregnancy, inability to use patient-controlled analgesia (PCA), patients’ refusal or inability to understand Cantonese.
Randomization &blinding
Patients were randomized to either the Lidocaine-group or the Placebo-group using a computer -generated random table (by a blinded research assistant) and an allocation ratio of 1:1. Allocation was concealed by enclosing assignments in sealed, opaque and sequentially numbered envelopes, which were opened only in the operation theatre. A “blinded” anaesthetist then prepared either lidocaine or saline according to the assigned group for the attending anaesthetist. Blinding of the healthcare professionals and the research personnel was maintained during the whole study period including all postoperative follow-ups.
Study intervention
For the Lidocaine-group, a bolus of intravenous lidocaine of 2mg/kg over 5 minutes was administered before skin incision. As for the Placebo-group, normal saline of equal volume was injected as bolus before skin incision.
Anesthesia and Perioperative Treatment
Study design
This was a prospective, single-center, double-blind, randomized, placebo-controlled trial. The trial was approved by the local university's Institutional Review Board (UW-18-26) and registered with an international clinical trials registry (www.clinicaltrials.gov, NCT0359776, https://clinicaltrials.gov/ct2/show/NCT03597776) before patient recruitment. Patients undergoing primary unilateral TKA were assessed for eligibility during preoperative screening visits. All eligible patients were informed about the study and written informed consent obtained. The study was conducted between January 2019 and January 2020 at the Duchess of Kent Children Hospital (DKCH), Hong Kong, in accordance with the ICH guidelines for Good Clinical Practice. CONSORT 2010 Statement was adopted as the reporting guideline. The full trial protocol is available to the reader upon request to the corresponding author.
Population
Eligible participants were all adults, aged 18-80 and with American Society of Anesthesiologists (ASA) physical status classification I-III, who were scheduled for primary unilateral TKA under spinal anaesthesia.
Exclusion criteria were defined as: any contraindications to or failed spinal anaesthesia, known intolerance or contraindication to local anaesthetics, paracetamol, non-steroidal anti-inflammatory drugs (NSAIDS) or opioids, single stage bilateral TKA / revision TKA, chronic pain other than knee pain, chronic use of opioids, substance abuse, cardiac disease (any degree of heart block / heart failure), any seizure disorder, psychiatric illness affecting pain perception; impaired renal function (defined as preoperative eGFR < 30ml /min /1.73 m2), impaired hepatic function, pregnancy, inability to use patient-controlled analgesia (PCA), patients’ refusal or inability to understand Cantonese.
Randomization &blinding
Patients were randomized to either the Lidocaine-group or the Placebo-group using a computer -generated random table (by a blinded research assistant) and an allocation ratio of 1:1. Allocation was concealed by enclosing assignments in sealed, opaque and sequentially numbered envelopes, which were opened only in the operation theatre. A “blinded” anaesthetist then prepared either lidocaine or saline according to the assigned group for the attending anaesthetist. Blinding of the healthcare professionals and the research personnel was maintained during the whole study period including all postoperative follow-ups.
Study intervention
For the Lidocaine-group, a bolus of intravenous lidocaine of 2mg/kg over 5 minutes was administered before skin incision. As for the Placebo-group, normal saline of equal volume was injected as bolus before skin incision.
Statistical Analysis
Sample size
Our null hypothesis was that there was no difference in the postoperative NRS pain score or total morphine consumption between the Lidocaine-group and the Placebo group.
In a local study that used a 10-point VAS pain scale, the mean (standard deviation) postoperative VAS pain score was around 3.1 (1.1) in patients who underwent primary TKA with a similar LIA regimen [9]. On this basis, the sample size necessary to detect at least a 30% difference with 80% probability and alpha < 0.05 was 38 patients in total (19 in each group). A 30% difference in NRS pain score was chosen because this has been shown to correspond to 'much improvement' in pain relief [10].
Regarding morphine consumption, a review of the literature on TKA in Chinese patients showed that the total does of post-operative morphine consumption was around 20mg (6.8) [11]. With an expected 25% reduction in morphine consumption, at an alpha set at 0.05 and 80% power, a total of 60 patients (30 in each group) would be required. Given that a 35% reduction in cumulative morphine consumption was reported in patients undergoing major abdominal surgeries [4], allowing some margins of errors, an expected 25% reduction in total morphine consumption was opted.
As a result, the minimal target sample size was 60. Considering potential drop-outs, we proposed to include 90 patients in total (45 in each group).
Data analysis
The statistical analyses were conducted using an intention-to-treat analysis with SPSS Statistics version 25 (IBM Corp., USA) and GraphPad Prism version 8.4.3 (GraphPad Software, Inc.,La Jolla, CA). Parametric data were presented as mean ± S.D using an unpaired Student t Test. Non-parametric data were presented as median + Inter-Quartile Range (IQR) and compared with a Man-Whitney U Test. Categorical data were presented as percentages and compared with Chi-Square Test or Fisher's exact test. A p-value of < 0.05 was considered significant.
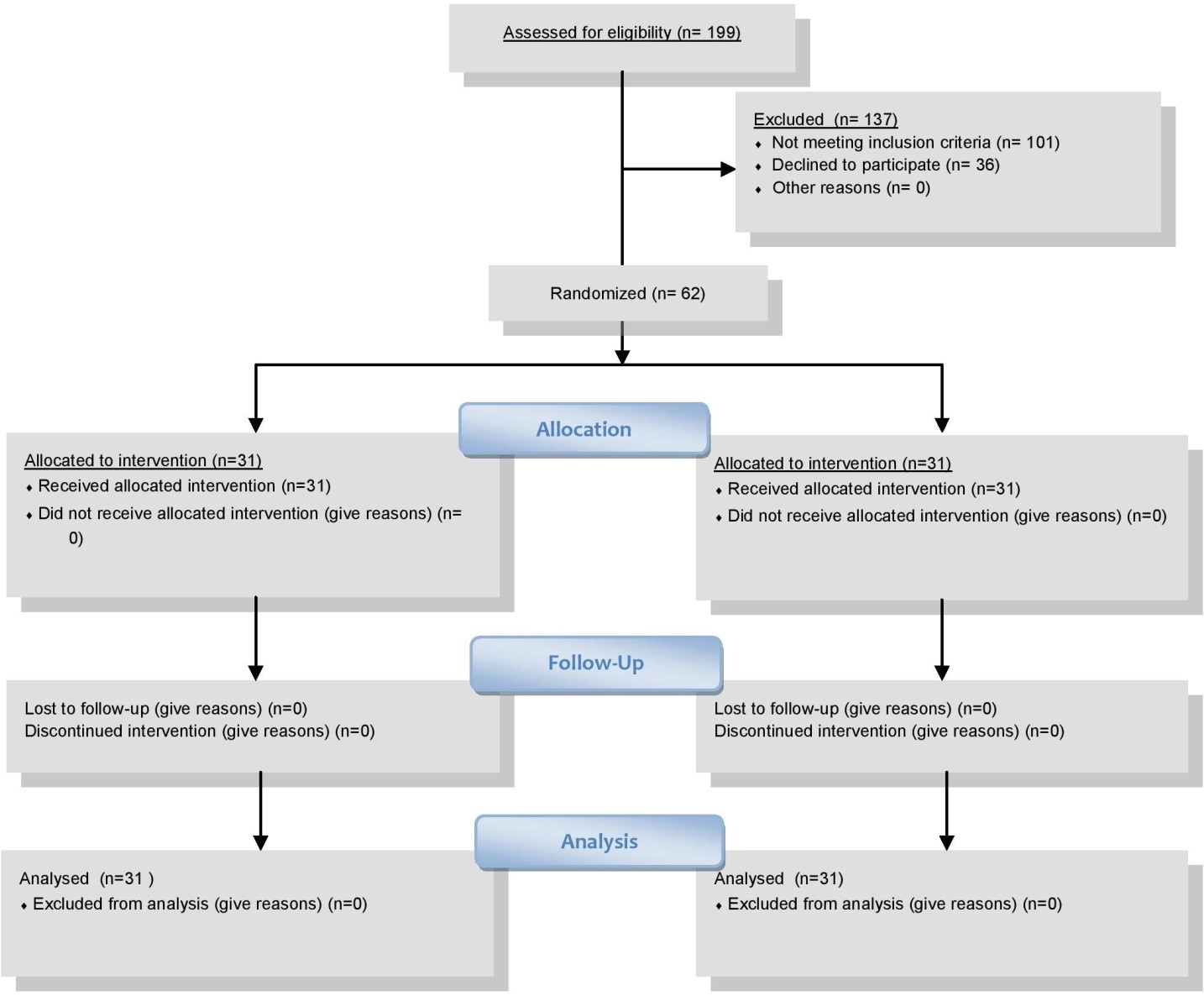
Figure 1: Patient’s recruitment.
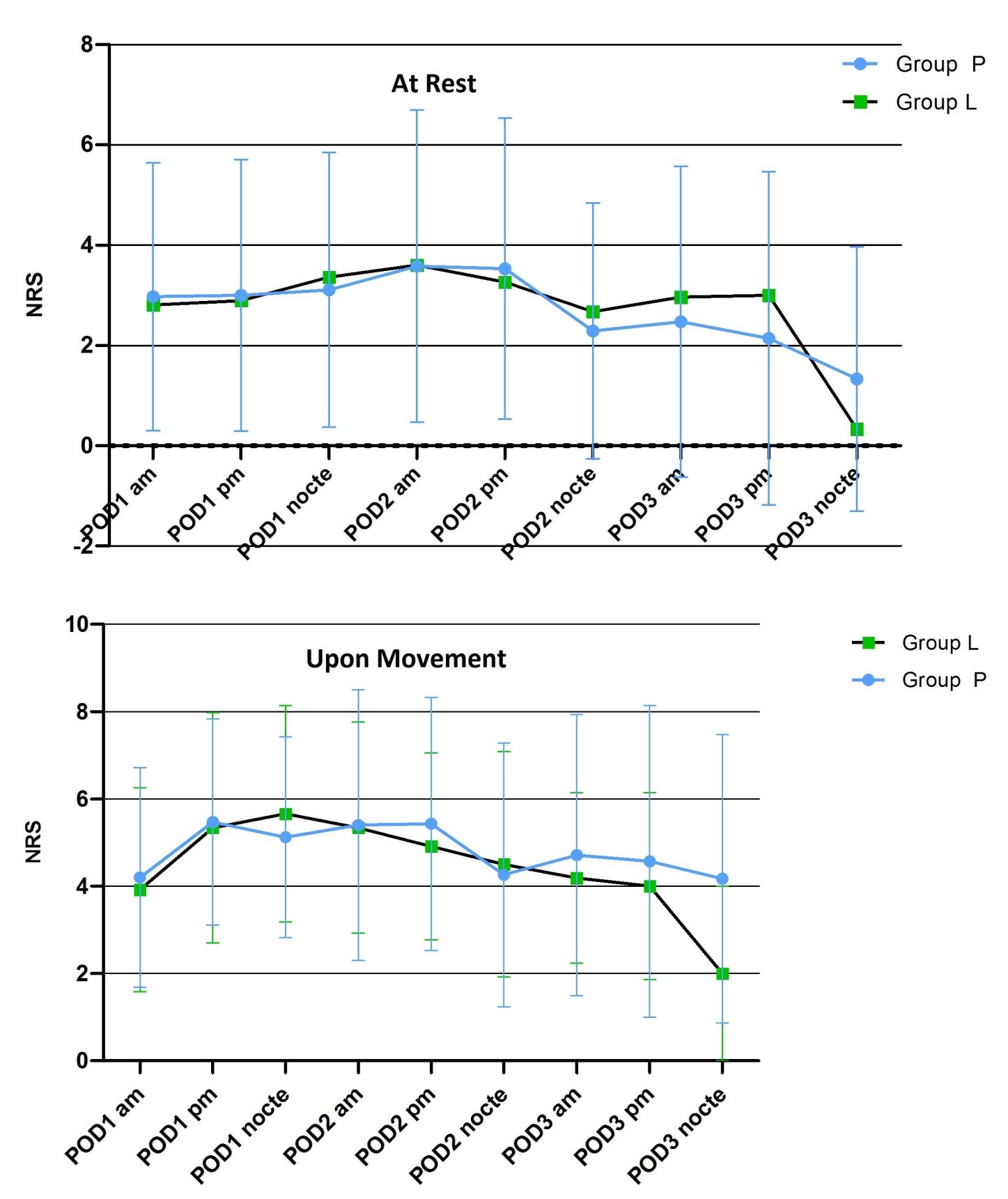
Figure 2: Initial post-operative pain scores at rest and upon movement.
Results
The recruitment chart is shown below (Figure 1). Among the 199 patients who were assessed, while 101 patients were excluded, 36 patients declined to participate. Thus, 62 patients were randomized to the Lidocaine-group (n = 31) or Placebo-group (n = 31). All enrolled subjects received the allocated intervention and were included in the intention-to-treat population. The drop-out was 0%. The whole recruitment started from January 2020 to December 2020
Preoperative Assessment and Intra-Operative Data
Patients’ baseline characteristics, intra-operative and PACU-related measures were all comparable (Table 1, 2).
Table 1: Pre-operative characteristics. Values in mean ± SD (range) or % (n).
Table 2: Intra-operative and PACU-related measures. Values in mean ± SD (range) or % (n).
Primary Outcomes
Although not statistically significant, NRS was generally lower in the Lidocaine-group compared to the Placebo-group on POD 1 morning and afternoon; for POD1 morning (P = 0.877; mean ± S.D. [95% C.I.] 2.81 ± 2.44 [1.93-3.69] vs. 2.97 ± 2.67 [1.99-3.94] NRS at rest; P = 0.827; 3.92 ± 2.33 [3.08 – 4.76] vs. 4.20 ± 2.52 [3.28 – 5.12] NRS upon movement ) and for POD1 afternoon (P = 0.741; 2.89 ± 2.92 [1.84 – 3.94] vs. 3.00 ± 2.71 [2.03 – 3.97] NRS at rest; P = 0.741; 5.34 ± 2.63 [4.39 – 6.29] vs. 5.47 ± 2.36 [4.62 – 6.32] NRS upon movement) (Figure 2, Table 3).
The cumulative PCA consumption (p=0.624, mean ± S.D. [95% C.I.] 2.36 ± 3.06mg [1.23-3.48] vs. 1.97 ± 3.13mg [0.82-3.11]) did not show any statistically significant difference between the 2 groups postoperatively (Table 3). Both groups did not receive any IMI rescue morphine or oral opioids.
Table 3: Post-operative pain. Values in mean ± SD (95% C.I.) or % (n).
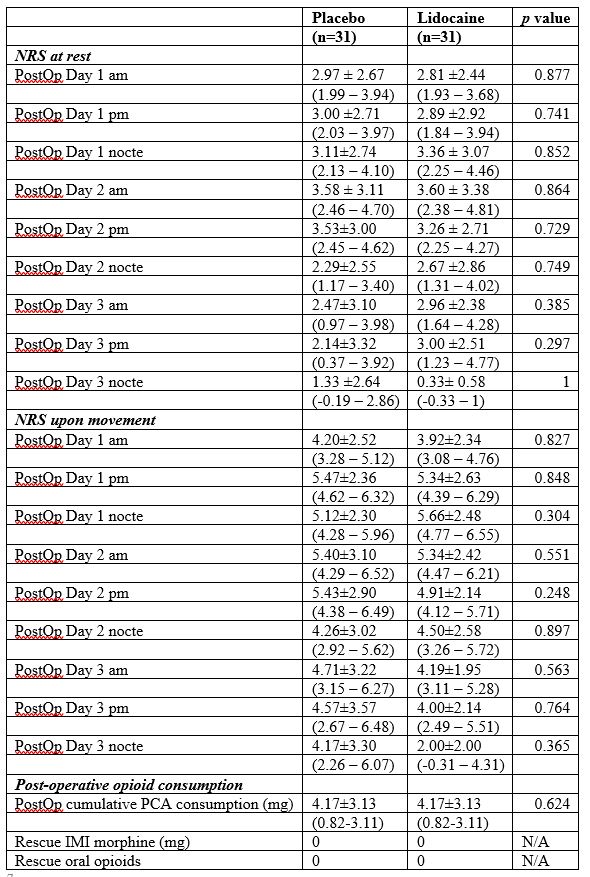
*am: morning; IMI: intramuscular injection; nocte: night; N/A: not applicable; NRS: numerical rating scale; pm: afternoon; PCA: patient-controlled analgesia; PostOp: post-operative
Secondary Outcomes
The Lidocaine-group had statistically significant shorter hospital LOS (p=0.0313, median [IQR] 3 days [3-4] vs. 4 days [3-4]) (Figure 3).
Though not statistically significant, the Lidocaine-group mobilised better with longer walking distance both on POD 1 (p = 0.325, mean ± S.D. [95% C.I.] 61.00 ± 21.67m [53.09 - 68.91] vs. 55.00 ± 25.02 m [45.87 - 64.14]) and POD 2 (p = 0.193, 70.00 ± 0.00 m [70.00-70.00] vs. 65.33 ± 13.56m [58.33 - 72.33]) (Table 4).
No statistically significant differences were detected in the other secondary outcomes such as active and passive ROM, the incidence of PONV, dizziness, return of bowel function, use of laxatives and antiemetics and various ADL scores. It is worth mentioning that no LAST or arrhythmia was reported in the Lidocaine-group (Table 4).
Table 4: Secondary Outcomes. Values in mean ± SD (95% C.I.) or % (n).
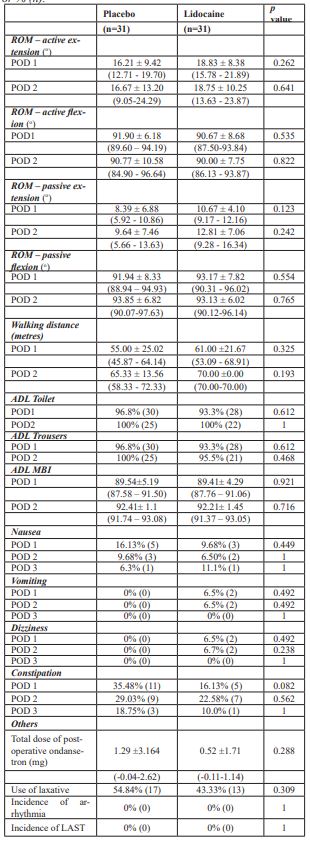
*ADL: activities of daily living; LAST: local anesthetic systemic toxicity; MBI: Modified Barthel Index; NBO: no bowel opening; POD: postoperative day; ROM: range of movement
Discussion
The initially planned sample size was 90 in total with drop-outs taken into consideration. However, this study was terminated prematurely due to the COVID-19 outbreak, which suspended all clinical trials and elective operations. Yet given a 0% drop-out rate, the minimal target sample size of 60 was still reached without compromising the power of the study.
In general, the NRS scores (both at rest and upon movement) and the cumulative morphine consumptions were low in both groups. None of the patients requested the administration of intramuscular morphine as rescue analgesics. These features reflected the efficacy of the standard perioperative analgesic regime used and might explain the low incidence of PONV and dizziness observed.
Of the two groups, the Lidocaine-group showed lower NRS at rest (POD 1 am & pm, POD 2 pm) and upon movement (POD 1 am & pm, POD 2 pm). The Lidocaine-group also demonstrated a longer walking distance on an average of 5metres on both POD1 and POD2.Although no statistically significant differences were observed for these outcomes individually, they may have cumulatively contributed to a statistically significant shorter hospital LOS of 1 day in the Lidocaine-group.
It has been shown that lidocaine exhibits a dose-dependent analgesic effect. In small doses (2 µg/ml), lidocaine inhibits ectopic impulse generation in peripheral nerves that are chronically injured; in moderate doses (5 µg/ml), lidocaine suppresses central sensitization and neuronal hyperexcitability; and in large doses (10 µg/ml), lidocaine exhibits general analgesic effects [12].
However, the routine use of LIA intraoperatively, which contained 300 mg of ropivacaine, significantly restricted the dose of lidocaine that could be administered without the concerns of causing LAST. Furthermore, continuous infusion lidocaine postoperatively in the ward would require the patient to be connected to an infusion pump, which may be inconvenient and may thereby hinder the patient’s mobilisation.
As such, a relatively low dose of lidocaine was opted, which may not be potent enough to produce clinically significant improvement in pain and other physical parameters.
Current studies and meta-analyses have suggested that IV lidocaine infusion provides significant analgesic effects and improves bowel function primarily in patients undertaken major abdominal surgeries [13-15]. However, such efficacy is not routinely demonstrated in patients having orthopaedic surgeries [16-17], including this study.
The reason for the discrepancy observed is not clear. Biochemically, the blood level of inflammatory mediators has been shown to be smaller and briefer after unilateral total hip replacement than after major abdominal surgeries [18]. Thus, for orthopaedic procedures, such as joint arthroplasty, which might possibly cause a lesser degree of inflammation , IV lidocaine might be less effective given anti-inflammation as one of its main mechanisms.
Clinically, patients undertaken orthopaedic surgeries are commonly encouraged to resume oral feeding and to mobilise through a more rigorous and structured rehabilitation program than patients undertaken major abdominal surgeries. These principles of ERAS, including multimodal opioid-sparing analgesia such as the regime used in this study, promote bowel movement. The effects of IV lidocaine on improving the bowel functions might therefore be less apparent in this patient population.
Moreover, the present study was powered solely for the primary endpoints. Significant difference on return of bowel function could possibly be unveiled after inclusion of a larger patient population. In fact, although not statistically significant, a lower incidence of constipation was observed in the Lidocaine group on POD 1-3 with less proportion of patients in the Lidocaine group in need of laxatives. For POD 1 (p = 0.082, 16.13% vs. 35.48%); POD 2 (p = 0.562, 22.58% vs. 29.03%); POD 3 (p = 1, 10.00% vs. 18.75%).
One limitation of this study was the relative low dose of a single bolus of IV lidocaine used. It is understood that a single bolus is less ideal than infusion in damping the inflammatory storms after TKA, which typically surge on POD 3 [19]. However, as discussed above, without the benefits of previous trials demonstrating the safety profiles of IV lidocaine in TKA, such was constrained by the concomitant use of LIA and the intent of promoting early mobilisation.
Secondly, the lidocaine concentrations and the cytokines levels were not measured in the present study. These parameters are believed to be important as they might explain the apparently varied effects of IV lidocaine between TKA and other major abdominal surgeries.
Thirdly, we did not study the long-term outcomes of peri-operative IV lidocaine administration. In fact, some available data now suggest that a brief period of perioperative lidocaine administration, despite the lack of short-term benefits, may improve long-term outcomes after complex spine surgeries [20] and breast surgeries [21].
Whilst this study failed to demonstrate a statically significant reduction in NRS and morphine consumption in the Lidocaine group, it suggested an improvement in other parameters that may have led to a shorter hospital stay.
Further research should therefore aim at studying the biochemical pattern of inflammatory mediators generated after TKA and thereby attempt to devise an optimal dosing regimen of IV lidocaine for patients undergoing unilateral primary TKA. IV lidocaine remains an affordable and widely available addition to the analgesic regime that is integral to the enhanced recovery and rehabilitation of TKA.
Conclusion
The Lidocaine-group had lower NRS at rest and upon movement on POD 1 am & pm and longer walking distance averaged 5 metres on both POD1& POD2. Although no statistically significant differences were observed for these outcomes individually, they may have cumulatively contributed to a statistically significant shorter length of hospital stay of 1 day in the Lidocaine-group.
Type of the Manuscript
Randomized; Double-blind; Placebo-Controlled Trial
Funding / Support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration
This research was presented in the Annual Scientific Meeting in Anaesthesiology 2020 and Evidence Based Perioperative Medicine - Asia 2020.
Acknowledgment
The authors acknowledge with thanks the contribution of Dr. H.T Chan for the general supervision and administrative work; Mr. SK Lin for data collection; Dr. CP Wong, Dr. DFOM Patricia, and the nursing staff at Duchess of Kent Children Hospital for their provision of clinical care to the patients enrolled in this study.
References
- Lee QJ, Mak WP, Wong YC. Mortality following primary total knee replacement in public hospitals in Hong Kong. HKMJ 2016; 22(3): 237–241
- Ganapathy S, Wasserman RA, Watson JT, et al. Modified continuous femoral three-in-one block for postoperative pain after total knee arthroplasty. Anesth Analg 1999; 89 (5): 1197-1202.
- Donnell RO, Dolan J. Anaesthesia and analgesia for knee joint arthroplasty. BJA Educ 2018; 18 (1): 8-15.
- Dunn LK, Durieux ME. Perioperative Use of Intravenous Lidocaine. Anesthesiology 2017; 126:729–737.
- Hahnenkamp K, Durieux ME, Hahnenkamp A, Schauerte SK. Local anaesthetics inhibit signaling of human NMDA receptors recombinantly expressed in Xenopus laevis oocytes: Role of proteinkinase C. Br J Anaesth 2006; 96:77-87.
- Hollmann MW, Gross A, Jelacin N, Durieux ME. Local anesthetic effects on priming and activation of human neutrophils. Anesthesiology 2001; 95:113-22.
- Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology 2000; 93: 858.
- Lahav M, Levite M, Bassani L, et al. Lidocaine inhibits secretion of IL-8 and IL-1b andstimulates secretion of IL-1 receptor antagonist by epithelial cells. ClinExpImmunol 2002; 127: 226-233.
- Fang YY, Lee QJ, Chang WY, Wong YC. Local infiltration analgesia in primary total knee arthroplasty. HKMJ 2019; 25(4): 279-286.
- Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003; 105(1-2): 151-157.
- Yue DB, Wang BL, Liu KP, Guo WS. Efficacy of multimodal cocktail periarticular injection with or without steroid in total knee arthroplasty. Chinese Medical Journal 2013; 126: 3851-3855.
- Abram SE, Yaksh TL. Systemic lidocaine blocks nerve injury induced hyperalgesia and nociceptor-driven spinal sensitization in the rat. Anesthesiology 1994; 80: 383-391.
- Sun Y, Li T, Wang N, Yun Y, Gan TJ. Perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum 2012; 55: 1183-1194.
- Marret E, Rolin M, Beaussier M, Bonnet F. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg 2008; 95: 1331-1338.
- Kranke P, Jokinen J, Pace NL, Schnabel A, Hollmann MW, Hahnenkamp K. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane Database Syst Rev 2015; 7: CD009642.
- Martin F, Cherif K, Gentili ME, et.al. E. Lack of impact of intravenous lidocaine on analgesia, functional recovery, and nociceptive pain threshold after total hip arthroplasty. Anesthesiology. 2008; 109: 118-123.
- Dewinter G, Moens P, Fieuws S, Vanaudenaerde B, Velde MVD, Rex S. Systemic lidocaine fails to improve postoperative morphine consumption, postoperative recovery and quality of life in patients undergoing posterior spinal arthrodesis. A double-blind, randomized, placebo-controlled trial. BJA 2017; 118(4): 576-585.
- Naito Y, Tamai S, Shingu K, et al. Responses of plasma adrenocorticotropic hormone, cortisol and cytokines during and after upper abdominal surgery. Anesthesiology, 1992; 77: 426-431.
- Moreschini O, Greggi G, Giordano MC, Nocente M. Postoperative physiopathological analysis of inflammatory parameters in patients undergoing hip or knee arthroplasty. Int. J. Tissue React. 2001; 23(4): 151-154.
- Terkawi AS, Sharma S, Durieux ME, Thammishetti S, Brenin D, Tiouririne M. Perioperative lidocaine infusion reduces the incidence of post-mastectomy chronic pain: A double-blind, placebo-controlled randomized trial. Pain Physician 2015; 18E139–46.
- Farag E, Ghobrial M, Sessler DI, Dalton JE, Liu J, Lee JH. Effect of perioperative intravenous lidocaine administration on pain, opioid consumption, and quality of life after complex spine surgery. Anesthesiology 2013; 119: 932-940.

