Triplet Chemotherapy with Cisplatin, Ifosfamide and 5-Fluorouracil in Advanced Anal Cancer. A Population Based Retrospective Analysis from the Danish Anal Cancer Group
Lisbeth Riber, Lars Henrik Jensen, Camilla Jensenius Skovhus Kronborg, Eva Serup-Hansen, Katrine Smedegaard Storm, Christina Glismand Truelsen, Karen Lycke Wind, Anders Jakobsen*, Karen-Lise Garm Spindler and Birgitte Mayland Havelund
Department of Oncology, Vejle Hospital, University Hospital of Southern Denmark, Denmark.
The Danish Centre for Particle Therapy, Aarhus University Hospital, Denmark.
Department of Oncology, Herlev and Gentofte Hospital, Denmark.
Department of Oncology, Aarhus University Hospital, Denmark.
Department of Experimental Clinical Oncology, Department of Oncology, Aarhus University Hospital, Denmark.
Received Date: 29/06/2021; Published Date: 16/07/2021
*Corresponding author: Anders Jakobsen, MD, DMSc, Department of Oncology, Vejle Hospital, University Hospital of Southern Denmark, Beriderbakken 4, 7100 Vejle, Denmark. E-mail: anders.jakobsen@rsyd.dk.
Abstract
Background: Squamous Cell Carcinoma of the Anus (SCCA) is a relatively rare malignancy with an annual incidence in the Western world of 0.5-1.7 per 100,000 citizens. Because of the low incidence, which is even lower when it comes to advanced disease, the knowledge on treatment options and clinical effect is limited.
Combination treatment with carboplatin and paclitaxel or cisplatin and 5-fluorouracil is internationally recommended as first-line treatment of metastatic or inoperable SCCA. However, complete remission is rare and the prognosis poor with a median survival of approximately 12 months. The combination of cisplatin, ifosfamide and 5-fluorouracil (CILF) represents an intensified treatment option and has been used for advanced SCCA in Denmark for several years, but its efficacy has not yet been evaluated, which was the aim of the present study.
Methods: We conducted a retrospective population-based study of all patients treated with palliative CILF for metastatic or inoperable recurrent SCCA in Denmark between October 2003 and October 2019 using Danish Hospital Registries.
Results: A total of 48 patients met the eligibility criteria. Median age at diagnosis was 57.4 years (range 41-75). The overall response rate was 39.6% (19 patients) of which 6.3% (three patients) had a complete response. Median progression-free and overall survival was 7.2 months (95% CI 3.4-8.6) and 13.3 months (95% CI 10.4-16.3), respectively.
Conclusion: This retrospective analysis reports a three-drug regimen with no added value compared to that of the standard regimens with cisplatin + 5-FU or carboplatin + paclitaxel.
Background
Squamous cell carcinoma accounts for 95% of cancers in the anal canal. It is a relatively rare malignancy with an annual incidence in the Western countries of 0.5-1.7 per 100,000 citizens [1]. The incidence is increasing, likely because of a higher prevalence of human papilloma virus (HPV) infection, which is expressed in more than 90% of squamous cell cancers of the anal canal (SCCA) [2]. Other known risk factors are HIV seropositivity, immunosuppression, smoking, passive anal intercourse, and age [3].
The vast majority of SCCA are diagnosed in localized stages and treated with radiation therapy (RT) alone or in combination with chemotherapy. However, more than 20% of patients develop advanced disease, either as local recurrence or distant metastases. The percentage is even higher in the case of large primary tumors or if spread to lymph nodes [4].
When salvage surgery is not possible, the treatment strategy is chemotherapy. Since the incidence of SCCA is low, and that of advanced disease even lower, the knowledge on treatment options and clinical effect is limited. The current literature is primarily based on small retrospective case series and case reports, and the types of chemotherapy used are largely extrapolated from the treatment of advanced squamous cell carcinomas of a higher incidence such as head and neck, lung, and cervical carcinomas [5-10]. Combination treatment with carboplatin and paclitaxel (or cisplatin and 5-fluorouracil (5-FU) has recently been internationally suggested as first-line treatment of metastatic or inoperable SCCA supported by the findings of a previous randomized study [11,12]. However, complete remission is rare and the prognosis poor with a median overall survival of approximately 12 months [2]. It is consequently relevant to investigate intensified chemotherapy combinations with the potential to increase the chance of complete remission and improved survival.
The combination of cisplatin, ifosfamide and 5-FU (CILF) represents an intensified treatment option and has been used for advanced SCCA in Denmark for several years. The regimen was primarily developed for the treatment of cervical cancer [13]. Its efficacy in advanced SCCA has not yet been evaluated, which was the aim of the present study.
Methods
Patients
The study was approved by the Danish Patient Safety Authority (3-3013-2447/1) and the Danish Data Protection Agency (1-16-02-66-18).
Patients were enrolled from the three centers in Denmark treating carcinomas in the anal canal, Aarhus University Hospital, Vejle University Hospital, and Herlev and Gentofte University Hospital. Patients treated between October 2003 and October 2019 were identified from the institutional registries by diagnosis and chemotherapy administered. Medical records were reviewed and patients with histologically confirmed SCCA having received palliative CILF were included. Demographics, extent of disease at time of decision on CILF treatment, previous treatments, response to treatment, and survival data were collected. Patients with advanced disease, who underwent a curable strategy, were not included. Hence, this cohort represents patients with the most severe prognosis.
Treatment
The CILF regimen consisted of cisplatin (37.5 mg/m2), ifosfamide (2.0 g/m2), 5-FU (500 mg/m2) + mesna (500 mg/m2 hour -2, +2 and +6) and leucovorin/calcium folinate (60 mg/m2) on days 1 and 2 every 3 weeks.
Statistics
The outcome measures were Progression Free Survival (PFS), Overall Survival (OS) and Objective Tumor Response Rate (OTRR).
Response was evaluated according to RECIST 1.1 (Response Evaluation Criteria in Solid Tumors 1.1) [14]. PFS was calculated from the first day of CILF treatment to progression or death. Patients with no event registered were censored on the date of the last follow-up.
OS was defined as the time between the first day of CILF treatment and date of death from any cause. Patients still alive were censored on the date of the last follow-up.
The distribution of patient characteristics and baseline variables are presented with descriptive statistics as average and percentages. PFS and OS were analyzed using the Kaplan-Meier method.
Table 1: Pre-treatment patient characteristics.
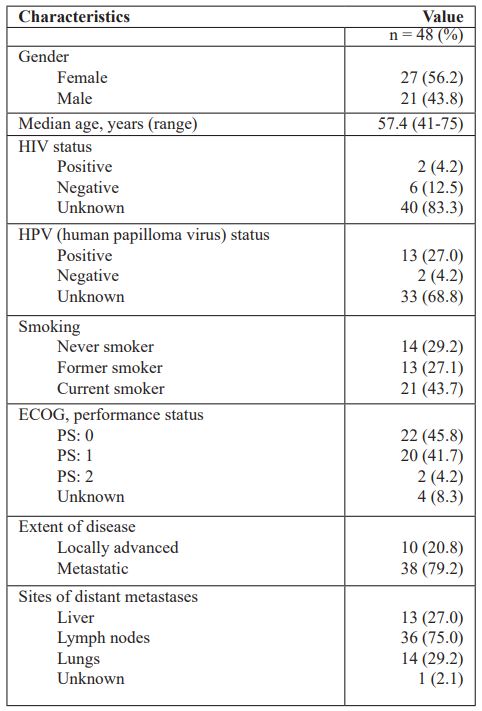
Table 2: Treatment characteristics
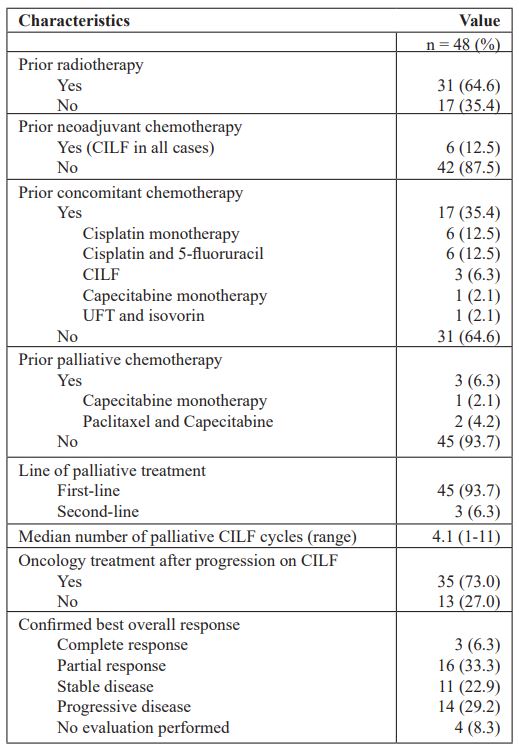
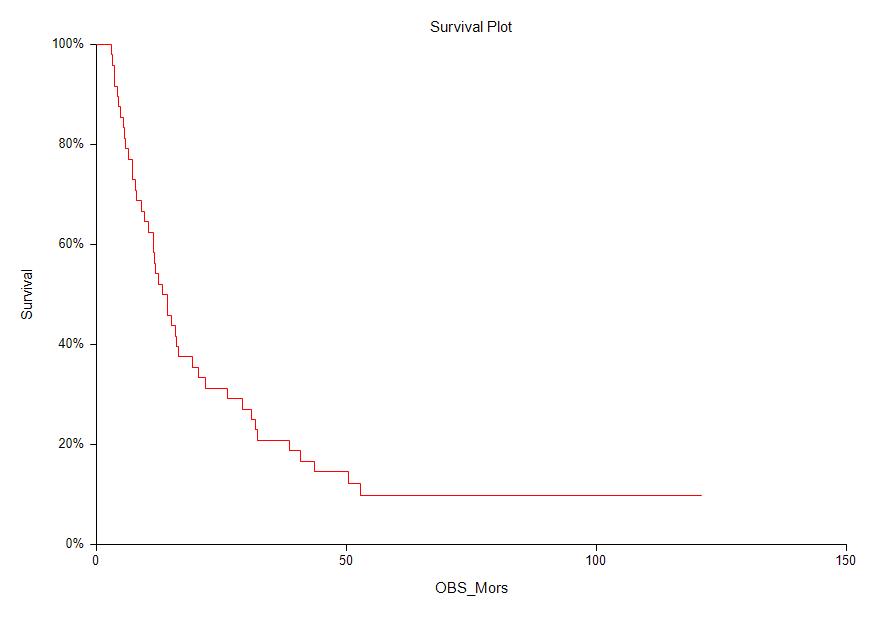
Figure 1: Overall survival
Results
Patient characteristics
A total of 48 patients met the eligibility criteria and were included in the study. Demographic and clinical characteristics are presented in Table 1. The median age at primary diagnosis of SCCA was 57.4 years (range 41-75) with a minor predominance of women (56.2%). Two patients were known HIV positive (4.2%) and 13 patients had known HPV positive tumors (27.0%). The status in relation to HIV and HPV was unknown in the majority of the cohort (83.3% and 68.8%, respectively). Twenty-two patients (45.8%) were in performance status (PS) 0, 20 (41.7%) in PS 1, and two (4.2%) were in PS 2. In four cases PS was not available. A total of 10 patients (20.8%) had locally advanced disease, whereas the remaining 38 patients (79.2%) presented with distant metastases. Lymph nodes were the most frequent metastatic site (75.0%).
Treatment characteristics
Table 2 shows treatment characteristics. The majority of the patients (31, 64.6%) had previously been treated with radiotherapy, 17 of which received concomitant chemotherapy. The most frequent radiosensitising regimens were cisplatin monotherapy (12.5%) and cisplatin + 5-FU (12.5%). Six patients (12.5%) had previously received induction chemotherapy in an attempt of a curative strategy. Only three patients (6.3%) had previously been treated with palliative chemotherapy (capecitabine monotherapy or paclitaxel + capecitabine). The median number of palliative CILF cycles was 4.1 (range 1-11). Thirty-five patients (73.0%) received oncology treatment after progression on CILF, i.e., radiotherapy, other palliative regimen as single agent or combination treatment (paclitaxel, capecitabine, cisplatin, 5-FU), reinduced CILF, and in one case immunotherapy with pembrolizumab.
Objective response was observed in 19 patients (39.6%) with either complete response (N=3, 6.3%) or partial response (N=16, 33.3%). An additional 22.9% obtained stable disease resulting in a disease control rate of 62.5% (Table 2).
Survival analysis
The median PFS was 7.2 months (95% CI: 3.4-8.6) and the median OS 13.3 months (95% CI: 10.4-16.3) (Figure 1).
Discussion
The present study describes an intensified treatment of advanced anal cancer by adding ifosfamide to cisplatin and 5-FU. The efficacy seems similar to that of the conventional regimens. It should be noted that the investigated patients represent a group with a dismal prognosis.
The treatment of advanced anal cancer represents a major challenge. The balance between treatment effect and toxicity is important, as the regimen cannot be considered curative. Most studies are based on small numbers of patients and the results should be interpreted with caution. A retrospective analysis of 77 patients treated with either cisplatin + 5-FU (55%), carboplatin + paclitaxel (31%) or alternative regimens (14%) reported a PFS of 7 months similar to the PFS presented here, and the same applies to a small study of 18 patients treated with carboplatin + paclitaxel reporting a median OS of 12 months11,15. A PFS of 12 months was found in a prospective single arm study evaluating a 3-drug regimen of docetaxel, cisplatin and 5-FU but with a high rate (70%) of grade 3 and 4 toxic events [4].
InterAAct is the first randomized study to investigate chemotherapy regimens in patients with advanced SCCA. Eligible chemotherapy-naïve patients (n=91) were randomized to cisplatin + 5-FU or carboplatin + paclitaxel. There was no difference in ORR (57% versus 59%), but more serious adverse events were found in the cisplatin + 5-FU arm (62%) compared to the carboplatin + paclitaxel arm (36%). Progression free survival was 5.7 months versus 8.1 months and OS 12.3 months versus 20 months (hazard ratio, 2.00, 95% CI: 1.15 to 3.47, P=0.014) in the cisplatin + 5-FU and carboplatin + paclitaxel arm, respectively, rendering carboplatin + paclitaxel a standard first line treatment [12].
Toxicity data were not available for the present study, but that of CILF treatment in advanced cervical cancer has been reported in detail previously [13]. The phase II trial found that of 194 CILF cycles administered there were 11 episodes (6%) of grade 4 neutropenia (WBC nadir <1000/µl) and 17 episodes (9%) of grade 4 thrombocytopenia (platelet nadir <25,000/µl). Ten patients (33%) developed distal sensory peripheral neuropathy of which nine were grade 1 and 2 and one was grade 3.
The present literature indicates that the current systemic treatment of advanced anal cancer is short of good results, and new treatment modalities are urgently needed. Checkpoint inhibitors such as nivolumab and pembrolizumab might have a role to play in the treatment of advanced SCCA. A phase II trial with 37 patients reported a response rate of 24% in patients previously treated with chemotherapy and the immunotherapy was well tolerated [16].
In addition to systemic treatment of patients with advanced SCCA, multidisciplinary interventions (e.g. surgery, radiofrequency ablation, etc.) have been evaluated by Eng et al. as a relevant strategy with the potential to improve overall survival in a selected group of patients, although the results should be interpreted with caution because of the risk of selection bias [11]. A multidisciplinary approach is likely to turn out as a valuable strategy to prolong tumor control and survival, at least in a selected group of patients with limited burden of disease.
The current study is limited by its retrospective nature and lack of toxicity data, but it represents real world data based on a population of approximately six million inhabitants.
Conclusion
The present study reports a three-drug regimen with no added value compared to that of the standard regimens with cisplatin + 5-FU or carboplatin + paclitaxel.
References
- Islami F, Ferlay J, Lortet-Tieulent J, Bray F, Jemal A. International trends in anal cancer incidence rates. Int J Epidemiol. 2017; 46(3): 924-938.
- Ghosn M, Kourie HR, Abdayem P, Antoun J, Nasr D. Anal cancer treatment: current status and future perspectives. World J Gastroenterol. 2015; 21(8): 2294-2302. doi:10.3748/wjg. v21.i8.2294
- Moureau-Zabotto L, Vendrely V, Abramowitz L, et al. Anal cancer: French Intergroup Clinical Practice Guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SNFCP). Dig liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2017; 49(8): 831-840. doi: 10.1016/j.dld.2017.05.011
- Kim S, Jary M, André T, et al. Docetaxel, Cisplatin, and 5-fluorouracil (DCF) chemotherapy in the treatment of metastatic or unresectable locally recurrent anal squamous cell carcinoma: a phase II study of French interdisciplinary GERCOR and FFCD groups (Epitopes-HPV02 study). BMC Cancer. 2017; 17(1): 574. doi:10.1186/s12885-017-3566-0
- Fountzilas G, Skarlos D, Athanassiades A, et al. Paclitaxel by three-hour infusion and carboplatin in advanced carcinoma of nasopharynx and other sites of the head and neck. A phase II study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol Off J Eur Soc Med Oncol. 1997; 8(5): 451-455. doi:10.1023/a:1008279503428
- Kelly K, Crowley J, Bunn PAJ, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: A Southwest Oncology Group trial. J Clin Oncol Off J Am Soc Clin Oncol. 2001; 19(13): 3210-3218. doi:10.1200/JCO.2001.19.13.3210
- Gibson MK, Li Y, Murphy B, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol Off J Am Soc Clin Oncol. 2005; 23(15): 3562-3567. doi:10.1200/JCO.2005.01.057
- Long HJ 3rd, Bundy BN, Grendys ECJ, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: A Gynecologic Oncology Group Study. J Clin Oncol Off J Am Soc Clin Oncol. 2005; 23(21): 4626-4633. doi:10.1200/JCO.2005.10.021
- Kaern J, Tropé C, Sundfoer K, Kristensen GB. Cisplatin/5-fluorouracil treatment of recurrent cervical carcinoma: a phase II study with long-term follow-up. Gynecol Oncol. 1996; 60(3): 387-392. doi:10.1006/gyno.1996.0059
- Green JA, Lainakis G. Cytotoxic chemotherapy for advanced or recurrent cervical cancer. Ann Oncol Off J Eur Soc Med Oncol. 2006; 17(1): x230-232. doi:10.1093/annonc/mdl265
- Eng C, Chang GJ, You YN, et al. The role of systemic chemotherapy and multidisciplinary management in improving the overall survival of patients with metastatic squamous cell carcinoma of the anal canal. Oncotarget. 2014; 5(22): 11133-11142. doi:10.18632/oncotarget.2563
- Rao S, Sclafani F, Eng C, et al. International Rare Cancers Initiative Multicenter Randomized Phase II Trial of Cisplatin and Fluorouracil Versus Carboplatin and Paclitaxel in Advanced Anal Cancer: InterAAct. J Clin Oncol Off J Am Soc Clin Oncol. 2020; 38(22): 2510-2518. doi:10.1200/JCO.19.03266
- Fanning J, Ladd C, Hilgers RD. Cisplatin, 5-fluorouracil, and ifosfamide in the treatment of recurrent or advanced cervical cancer. Gynecol Oncol. 1995; 56(2): 235-238. doi:10.1006/gyno.1995.1038
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45(2): 228-247. doi: 10.1016/j.ejca.2008.10.026
- Kim R, Byer J, Fulp WJ, Mahipal A, Dinwoodie W, Shibata D. Carboplatin and paclitaxel treatment is effective in advanced anal cancer. Oncology. 2014; 87(2): 125-132. doi:10.1159/000361051
- Morris VK, Salem ME, Nimeiri H, et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017; 18(4): 446-453. doi:10.1016/S1470-2045(17)30104-3

