In Physically Active Patients Predisposed to Bone-Muscle Attrition Receiving Androgen Deprivation Therapy for Prostate Cancer Treatment, Prospective MRI Evaluation Does Not Demonstrate Significant Muscle Alteration
Bayan N Mogharrabi, Uma J Thakur1, Yin Xi, Orhan Oz, Naim Maalouf, Craig D Rubin and Avneesh Chhabra*
Department of radiology at the University of Texas at Southwestern Medical Center
Department of radiology at the University of Texas at Southwestern Medical Center
Department of radiology at the University of Texas at Southwestern Medical Center
Department of radiology at the University of Texas at Southwestern Medical Center
Department of internal medicine at the University of Texas at Southwestern Medical Center
Department of internal medicine at the University of Texas at Southwestern Medical Center
Department of radiology at the University of Texas at Southwestern Medical Center
Received Date: 26/06/2021; Published Date: 14/07/2021
*Corresponding author: Avneesh Chhabra M.D, Department of radiology at the University of Texas at Southwestern Medical Center (5323 Harry Hines Blvd, Dallas, TX 75390), Email: Avneesh.Chhabra@UTSouthwestern.edu, Mobile no: 214-648-2122
Abstract
Introduction/aims: To prospectively determine the effects of Androgen Deprivation Therapy (ADT) on muscle mass and intramuscular proton diffusion on 3-Tesla MRI. We hypothesized that androgen deprivation therapy will cause a significant decrease in muscle mass and increase in Apparent Diffusion Coefficient (ADC).
Methods: A consecutive series of 16 physically active patients with proven prostate cancer treated with androgen deprivation therapy were prospectively recruited. MRI was obtained at 3 time points at two sites (gluteal and thigh regions) using a torso XL coil and a combination of 3-dimensional (3D) T2 Dixon and DWI sequences. Two trained readers measured muscle areas on axial reconstructions from 3D imaging and apparent diffusion coefficient of bilateral gluteus medius, iliopsoas and quadriceps muscles at fixed points. Linear mixed model was used to analyze the trends in each measurement along 3 time-intervals.
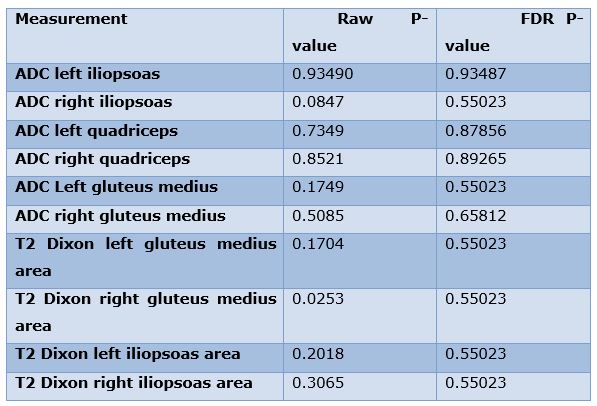
Result: Agreement between the readers was good to excellent for all measurements except area measurements of the bilateral iliopsoas muscles. On further mixed modeling, false discovery rate adjusted p-value did not show significant linear trend across any of the measurements (p>0.05). During at least six-month follow-up period, the patients denied any falls or fractures.
Discussion: In physically active men, androgen deprivation therapy in prostate cancer patients does not result in significant morphologic changes in pelvis and thigh muscle area or functional intramuscular diffusion in the short-term.
Keywords: Prostate Cancer, Fracture, Magnetic Resonance Imaging, Muscle, Diffusion-weighted Imaging
Introduction
The Global Cancer Observatory identified Prostate Cancer as the 2nd most commonly diagnosed cancer and 5th most deadly in men worldwide [1]. Androgens are important for the growth of the prostate gland, and men with elevated testosterone levels are shown to have an increased risk for developing prostate cancer [2]. Androgen deprivation therapy (ADT) has been used as a standard of care treatment for prostate cancer amelioration and it has shown to reduce adult prostatic epithelial cell growth and improve the quality of life in such patients [3,4]. Although ADT has been useful in the treatment of prostate cancer, it causes many adverse effects such as decreased bone density as confirmed by DEXA (dual energy X-ray absorptiometry) scans and increased fracture risk. Since the muscle-bone unit works in tandem and testosterone receptors are expressed on the myocytes, it has been proposed that myopenia and/or altered muscle function may predispose to a risk of increased falls and/or fractures [5]. However, to our knowledge, the effects of ADT on muscle mass or diffusion characteristics have not been studied to date. Our project aimed to prospectively determine the effects of ADT on the magnitude of intramuscular diffusion and muscle mass in physically active patients undergoing treatment for prostate cancer. We hypothesized that ADT will cause a significant decrease in muscle mass and increase Apparent Diffusion Coefficient (ADC) during the 6-month follow-up after initiation of ADT.
Methods
This study was a prospective cohort evaluation and all patients provided the informed consent. Institutional review board guidelines were followed, and the MRI studies were reviewed in a HIPAA compliant manner.
Patients
A consecutive series of 16 physically active patients who presented to our University hospital for the treatment of first-time diagnosis of prostate cancer from the October 2017- February 2020 were included. The patients were maintaining activities of daily living, exercised regularly (3x a week) and were counseled to keep active lifestyles. The inclusion criteria were- men between the ages of 56-89 years, known prostate cancer, patients due to receive ADT and no contraindications for MRI.
MRI acquisition:
All studies were scanned on a 3T (Tesla) MR scanner (Ingenia, Philips) from L2 to proximal thigh using a torso XL coil and spine coils. A combination of 3-dimensional (3D) T2 Dixon and diffusion weighted imaging (DWI) sequences the patients received MR imaging at 3 time points: before ADT (time 0), and at 6 weeks and 6 months following ADT initiation, respectively. (Table 1).
Data analysis
A fellowship trained musculoskeletal (MSK) radiologist and another trained medical student reader under the close supervision of a different fellowship trained musculoskeletal radiologist performed all the measurements. Eight MRI scans of the total 48 scans were reviewed together by the two readers for the initial training. A week after the initial training, both readers reviewed the imaging independently and recorded the measurements blinded to each other’s reads and blinded to the prostate disease or ADT status. The muscle areas were measured on the axial isotropic reconstructions from 3D volume imaging and Apparent Diffusion Coefficients (ADC) were obtained from the corresponding slice axial DWI maps. The muscles were evaluated at fixed points and measurements were obtained from bilateral gluteus medius, iliopsoas and quadriceps muscles. The gluteus medius and iliopsoas were measured in the transverse plane at the level of the synovial part of the sacro-iliac joint and the quadriceps muscles were measured immediately below the lesser trochanter to ensure standardization. Image reconstruction and measurements were performed using HOROS software and employing a free hand ROI incorporating the whole muscle with careful exclusion of the regional fatty tissue (Figure.1 and Figure. 2). The data was recorded on a Microsoft excel spreadsheet. Qualitatively, muscle edema and fatty infiltration was assessed by a different senior MSK radiologist.
Statistics
Being a pilot prospective study, no power calculation was attempted. Agreement between the two readers was assessed using intraclass correlation coefficient (ICC) through one-way mixed effects models. ICC were interpreted as 0.0-0.2: poor; 0.2-0.4: fair; 0.4-0.6: moderate; 0.6-0.8: good; and greater than 0.8: excellent. Linear mixed models were used to test the difference in each measurement among the respective 3-time intervals. False discovery rate was reported. P<0.05 was considered statistically significant.
Table 1: Magnetic Resonance Imaging Parameters
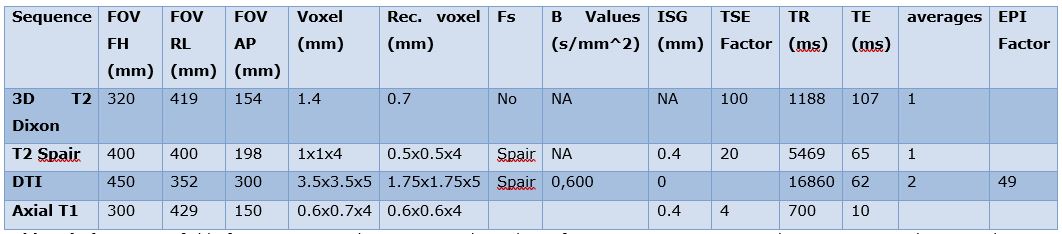
Abbreviations: FOV, field of view: Rec. voxel, reconstructed voxel; Fs, fat suppression; ISG, Inter-slice Gap; TSE, Turbo spin echo; TR, repetition time; TE, echo time; DTI, diffusion tensor imaging; ms, milliseconds; mm, millimeters; SPAIR, spectral attenuated inversion recovery; EPI factor, Echo-planar im
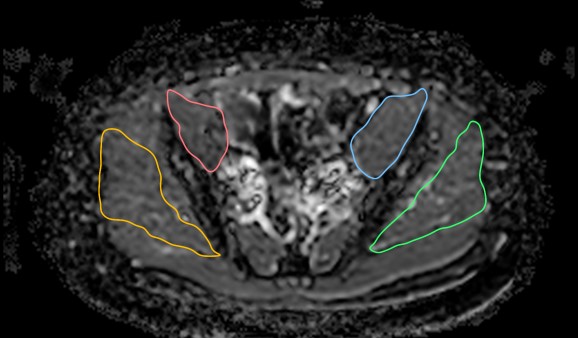
Figure 1- ADC image of Gluteus medius and iliopsoas with measurements
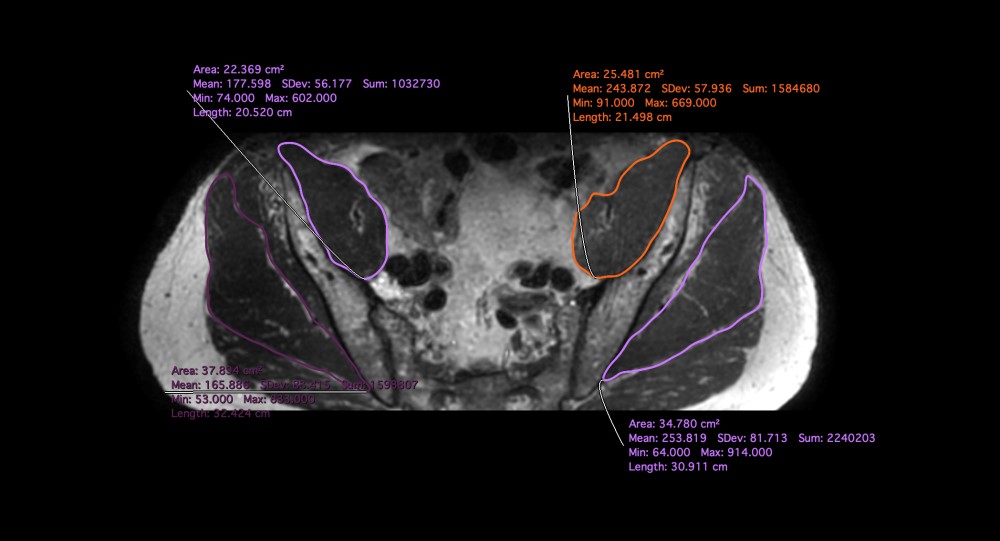
Figure 2- Axial reconstruction from 3D T2 Dixon showing measurements of gluteus medius and iliopsoas
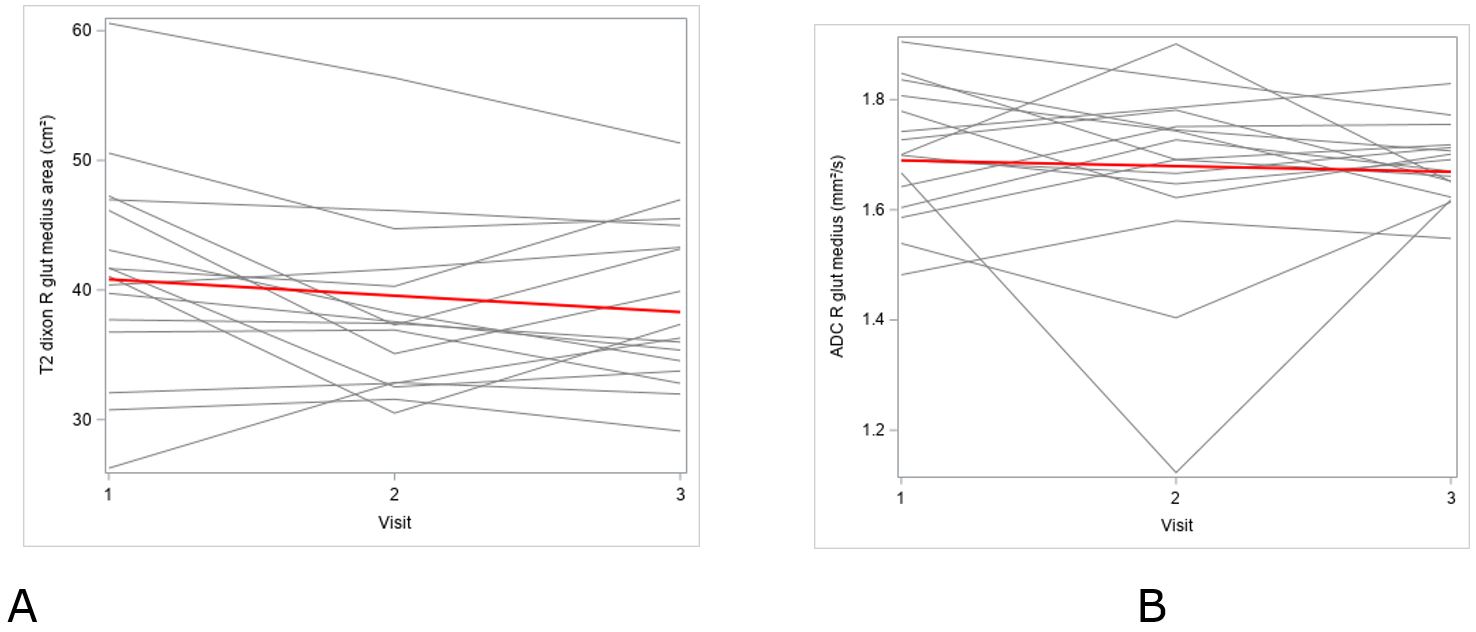
Figure 3- Measurements of T2-dixon area (A) and ADC (B) of right gluteus medius across three time points, which revealed no linear trend across any of the measurements.
Results
There were 16 male patients (13 Caucasian, 3 African American) with ages ranging from 56-89 years (median 67 years), BMI median of 29.8 kg/m2, and PSA 4.57 (0.94, 7.31) ng/ml. The men exercised 3 times a week on average with no substantial change in habits before or after ADT therapy. There was a total of 48 MRIs (16 patients at 3 time points). All patients completed the whole imaging examinations. Serologically, as measured using standard institutional assays, the testosterone levels declined by week six in all patients that persisted on the 24-week check-up, confirming complete ADT response on the hormonal status. None of the patients reported a fall or fracture during the 6-month follow-up visit. Qualitatively, there was no edema like signal or noticeable fatty infiltration of muscles on the water map and in-phase maps of T2 Dixon imaging, respectively.
Inter-reader correlation
Among the 10 variables recorded, all measurements showed excellent inter-reader correlations, except for the areas of the left and right iliopsoas on T2 Dixon, which showed good correlation, confirming the validity of the measurements. The details of ICC and confidence intervals are depicted in Table 2.
Table 2: Inter-reader (ICC) correlations and respective confidence intervals.
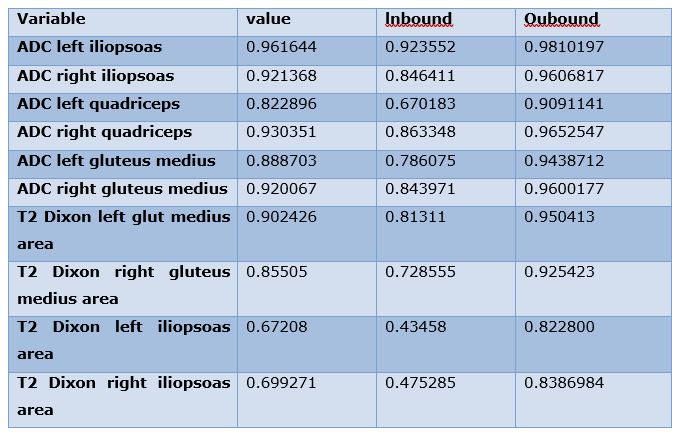
Table 3: Linear trends across 3-time lines among muscle area and ADC measurements
Discussion
It has been shown that patients undergoing ADT therapy for the treatment of prostate cancer incur a loss of muscle mass and consequently muscle strength [6]. We didn’t find any significant change in muscle loss or alteration in diffusion characteristics. Other studies have revealed that loss of muscle density and attenuation as well as increase in fatty infiltration occurs in patients after ADT [7]. Ultimately, these deleterious effects on muscle could cause patients on ADT to exhibit a drastic change in physical function and quality of life [8]. Contrary to the reviewed literature, our study revealed no observable trend towards muscle loss as assessed by 3-D MR imaging. One difference in our cohort was that the men were physically active. Furthermore, prior research has indicated that there is an increase in fracture risk, loss of bone mineral density and increase in fall risk and frailty [9-11]. Our work did not investigate direct impact of ADT on bone properties but on the 6-month follow-up, none of our patients reported any falls or fractures.
This exploratory study results are limited by a small prospectively acquired sample with a risk of type II error, however ADT response was confirmed by suppression of serum testosterone levels. Larger studies in the future may confirm the results from this pilot study. A 2019 study shows that in patients undergoing ADT therapy for the treatment of prostate cancer, exercise has a protective effect on muscle mass, which could explain the lack of muscle loss in this work [12]. Another possibility is that there could be a change in muscle quality/microscopic composition; future studies can aim to use different MR sequence to analyze various other muscle properties, such as quantitative fat fraction and elasticity analysis. However, we did study the intramuscular diffusion. The muscle ADC values did not show significant trend towards increased or decreased diffusion. Microscopic muscle edema or altered architecture was expected but the results did not substantiate our hypothesis.
The inter-reader analysis was good to excellent on all measurements. Uniform scanning using a single 3-T research scanner and isotropic 3D Dixon imaging facilitated excellent imaging resolution for standardized axial reconstructions and measurements.
The results may also be heavily influenced by the short-term follow-up, i.e., results are reported at 6 months of ADT, as previous studies analyzing the effects of ADT on bone health have reported these adverse changes occurring after one year [13]. Our results cannot be generalized to all prostate cancer patients undergoing ADT as none of our patients had metastasis and they all indicated good physical activity.
In future, the results of this pilot prospective study can be instantiated with larger cohort studies and comparison between physically active versus sedentary men could be performed. The results may guide institution of specific muscle strengthening protocols. We did not study muscle fat fraction as part of this article and such analysis may provide additional insights into temporal changes in muscle pathophysiology.
Conclusion
This pilot prospective study showed that there is no observable trend in muscle area loss or altered intramuscular diffusion in physically active prostate cancer patients undergoing ADT. The study establishes that MRI markers of skeletal muscles are not susceptible to ADT therapy in the short-term and suggests that specific muscle strengthening protocols might not be critical in physically active men during the follow-up visits to prevent future falls.
Author Contributions
Bayan Mogharrabi B.S: Data acquisition, drafting of the article, and gave final approval of the article.
Uma J. Thakur M.D.: Data acquisition, revision of the article, and gave final approval of the article.
Yin Xi Ph.D.: Data interpretation and analysis, revision of the article and gave final approval of the article.
Orhan Oz M.D.: Conception and design of the project, revision of the article, and gave final approval of the article.
Naim Maalouf M.D.: Conception and design of the project, revision of the article, and gave final approval of the article.
Craig D. Rubin M.D.: Conception and design of the project, revision of the article, and gave final approval of the article.
Avneesh Chhabra M.D.: Conception and design of the project, drafting and revision of the article, gave final approval of the article, and is the designated “guarantor”.
Funding: The Endowed Professors’ Collaborative Research Support from the Charles Y.C. Pak Foundation
Disclosures: Avneesh Chhabra serves as a consultant to ICON Medical and Treace Medical Concepts, Inc. Avneesh Chhabra also receives royalties from Jaypee and Wolters. No other author has any disclosures.
Conflicts of interests: None of the authors has any conflict of interest to disclose.
Grant Information: The Endowed Professors’ Collaborative Research Support from the Charles Y.C. Pak Foundation (Funding)
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394-424.
- Shaneyfelt T, Husein R, Bubley G and Mantzoros CS. Hormonal predictors of prostate cancer: a meta-analysis. J Clin Oncol 2000; 18: 847-853.
- Sharifi N, Gulley JL and Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA 2005; 294: 238-244.
- Huggins c, stevens re, jr. And hodges cv. Studies on prostatic cancer: ii. The effects of castration on advanced carcinoma of the prostate gland. Archives of Surgery 1941; 43: 209-223.
- Patil T and Bernard B. Complications of Androgen Deprivation Therapy in Men With Prostate Cancer. Oncology (Williston Park) 2018; 32: 470-474, CV473.
- Østergren PB, Kistorp C, Bennedbæk FN, Faber J, Sønksen J and Fode M. The use of exercise interventions to overcome adverse effects of androgen deprivation therapy. Nature Reviews Urology 2016; 13: 353-364.
- Chang D, Joseph DJ, Ebert MA, Galvão DA, Taaffe DR, Denham JW, Newton RU and Spry NA. Effect of androgen deprivation therapy on muscle attenuation in men with prostate cancer. Journal of Medical Imaging and Radiation Oncology 2014; 58: 223-228.
- Storer TW, Miciek R and Travison TG. Muscle function, physical performance and body composition changes in men with prostate cancer undergoing androgen deprivation therapy. Asian J Androl 2012; 14: 204-221.
- Newton RU, Galvao DA, Spry N, Joseph D, Chambers SK, Gardiner RA, Wall BA, Bolam KA and Taaffe DR. Exercise Mode Specificity for Preserving Spine and Hip Bone Mineral Density in Prostate Cancer Patients. Med Sci Sports Exerc 2019; 51: 607-614.
- Winters-Stone KM, Moe E, Graff JN, Dieckmann NF, Stoyles S, Borsch C, Alumkal JJ, Amling CL and Beer TM. Falls and Frailty in Prostate Cancer Survivors: Current, Past, and Never Users of Androgen Deprivation Therapy. J Am Geriatr Soc 2017; 65: 1414-1419.
- Shahinian VB, Kuo YF, Freeman JL and Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 2005; 352: 154-164.
- Gazova A, Samakova A, Laczo E, Hamar D, Polakovicova M, Jurikova M and Kyselovic J. Clinical utility of miRNA-1, miRNA-29g and miRNA-133s plasma levels in prostate cancer patients with high-intensity training after androgen-deprivation therapy. Physiol Res 2019; 68: S139-S147.
- Nguyen PL, Alibhai SM, Basaria S, D'Amico AV, Kantoff PW, Keating NL, Penson DF, Rosario DJ, Tombal B and Smith MR. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015; 67: 825-836.

