The Correlation between Placental Weight and Foetal Outcome in a Tertiary Health Facility in Southern Nigeria
Emechebe CI*, Ekanem E, Ukaga JT, Njoku CO
Department of Obstetrics and Gynecology, University of Calabar Teaching Hospital (UCTH), Calabar, Nigeria
Received Date: 16/06/2021; Published Date: 30/06/2021
*Corresponding author: Dr. Emechebe CI, Department of Obstetrics and Gynecology, University of Calabar Teaching Hospital (UCTH), Calabar, Nigeria. E-mail: newlifecj2000@gmail.com
Abstract
Background: Placenta is a vital feto-maternal organ for promoting pregnancy, foetal growth and development. Placenta varies in weight, size, thickness, form and consistency. The weight of the placenta is functionally significant because it is related to villous surface area and to foetal metabolism. Gross examination of placenta after delivery may provide a useful insight into foetal weight, newborn and maternal complications of pregnancy.
Objectives: To determine the mean placental weight in our environment and also, to determine the correlation between the placental weight and the neonatal outcome in term pregnancies in Calabar.
Materials and Methods: This was cross-sectional study of 300 women conducted at university of Calabar Teaching Hospital (UCTH). Participants in the study were women with singleton pregnancies who delivered either by vaginal delivery or caesarean section at term. Examination and weighing of placenta were done at delivery for placental weight. Foetal outcome that was recorded included birth weight, sex, length of the newborn and Apgar score at 5th minute. Maternal age, marital status and parity were also obtained. Data obtained were analyzed using the statistical package for Social Sciences (SPSS) version 20. Level of significance was set at p-value less than 0.05. Data was presented in tables and graphs.
Results: The mean placental weight was 652 ±152g and ranged from 250g-1,200g. The mean foetal birth weight was 3.309 ±0.522kg and ranged from 2.0-5.9kg. The mean placental weight to birth weight ratio was 1: 5.08. The mean gestational age at delivery was 38.94 ±1.33weeks while the mean length of the neonate was 49.79 ±2.66cm. There was a corresponding increase in placental weight with increase in neonatal birth weight and length of the neonate.
Conclusions: There was a positive association between placental weight and foetal outcome at birth. Placental weight was positively correlated with birth weight and length of the neonate. Critical examination and weighting of the placenta immediately after delivery should be done to determine the well being of the newborn.
Keywords: placental weight, birth weight, length of neonate, foetal outcome
Introduction
The capacity of foetus to grow and develop during pregnancy depends on the quality and function of the developed placenta [1]. This is because normal developed placentae have several functions during pregnancy such as nutritive, excretory, respiratory, endocrine and barrier action in preventing harmful substances from passing to the fetus [2]. The placental weight is about one sixth of the normal weight of the newborn baby [1]. Some studies have examined the relationship of placental size to the neonatal birth weight at term and concluded that placental weight was linked with pregnancy outcome [1,3]. The normal mature human placenta is rounded, flattened, discoid organ, 15-20cm in diameter and 2-4cm thick. It weighs 500-600grams [2]. Placentae vary in weight, size, thickness, form and consistency. Studies revealed that both high and low placental weights correlated with a poor perinatal outcome such as low Apgar score, medical complications of pregnancy, respiratory distress syndrome and perinatal death [1,4]. For example, they are heavier than normal in maternal conditions like syphilis, diabetes, hydrops fetalis, rhesus isoimmunization and severe anemia [2]. Small placental sizes may be seen in conditions like trisomy’s and maternal hypertension. The central pathology to the development of pre-eclampsia lies in the placenta [5]. In pre-eclampsia, there is a failure of cytotrophoblast invasion of the uterine spiral arteries, which remains superficial and does not reach the myometrial level. Thus, the spiral arteries remain as undiluted and high resistance vessels, and they respond to vasomotor substances like angiotensin II and noradrenaline [5]. The failure of trophoblast invasion is also, seen in other conditions where there is placental insufficiency such as pregnancy induced hypertension, intrauterine growth restriction and chronic hypertension. Also, Barker et al reported that distorted growth of the placenta was a predictor of maternal medical diseases including cardiovascular disease, hypertension and diabetes mellitus [6]. Other factors such as socioeconomic status, race, harmful habits and occupation also affect the placental weight [1].
Cautious inspection of the placenta after delivery can give insight concerning the intrauterine environment of the fetus before delivery. Recent studies on fetal birth weights show that fetal birth weights at term have increased over time [7,8]. There is a positive correlation between fetal weight and placental weights in some studies [1,3]. This study in addition to determining the correlation between placental weight and fetal weights, will also, determine the correlation between the placental weight and other fetal outcome such as fetal length, fetal sex and Apgar score. The placenta can be weighed with membranes and cord attached, but the standard approach is to weigh the placenta after the extra placental membranes and the umbilical cord are trimmed from the disk [1]. This limits the measurement to the weight of the placental disk, the actual nutrient exchange part of the placenta. However, Leary et al suggested that trimmed and untrimmed placental weights are exchangeable, based on their high correlation [9].
The placental weights examination and measurement are of great clinical relevance and is associated with a wide range of unfavourable obstetric outcome and this study was aimed to determine the correlation between placental weight and foetal outcome. There are few documented local studies of placental weight and foetal outcome [1,3]. Though the pathogenesis of variability of placental size remains largely unclear, this study would provide information about the placental weight and its association with adverse obstetrics outcome in our locality. Additionally, it will also guide further studies on the subject in this environment and enrich local content of literature. It will also, provide a basis for future best practices in maternal and prenatal care and its effects on neonatal outcome will help in future care of new born to improve neonatal outcome.
Methodology
This study was a prospective cross-sectional study carried out at the labour ward and theatre of the Department of Obstetrics and Gynaecology, University of Calabar Teaching Hospital (UCTH), Calabar from February 15th to July 14th 2016. The target population were pregnant women delivering either vaginally or by caesarean section in this facility.
Inclusion criteria were primigravida or multigravida with singleton pregnancies who had either vaginal delivery or caesarean section between 37 and 42 completed weeks.
Exclusion criteria were pregnancies complicated by intrauterine foetal death, multiple gestations, congenital malformations of foetus, diabetes, preeclampsia, chorioamnionitis, preterm labour and intrauterine growth restriction.
Ethical approval was obtained from the ethics and research committee of the Hospital before commencement of the study. Informed consent was obtained from clients after being recruited for the study.
Data were obtained from participants using a pre-tested questionnaire, new born weighing scale and measuring tape calibrated in centimetres. The new born weighing scale was a standard analogue Way master (England) scale corrected for zero error. This was used to measure the neonatal and placental weight after delivery.
The socio-demographic characteristics obtained include age, marital status, parity, last menstrual period and gestational age in weeks. The placentae were examined under running water to remove blood clots and the placental weight measured using a weighing scale and recorded in grams. Foetal parameters that were recorded after delivery include Apgar score at 5 min, weight of the new born, sex and length of new born.
Data obtained were analysed using the Statistical Package for Social Sciences (SPSS) version 20. The test of significance was done with Pearson’s correlation coefficient. Level of significance was set at p-value less than 0.05. Data were presented in tables and chart.
Table 1: The mean and range of the characteristics of the study group
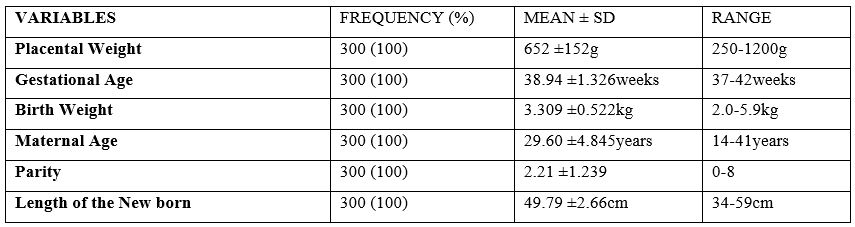
The male foetuses had slightly larger mean placental weight than female foetuses though the difference was not statistically significant (663 ±141g vs 640 ±164g; P-value=0.203) as shown in table 2. The mean placental weight increased with increase in birth weight and there was statistically significant different between placental weight and birthweight.
Table 2: The socio-demographic characteristics in relation to mean placental weight in the study population
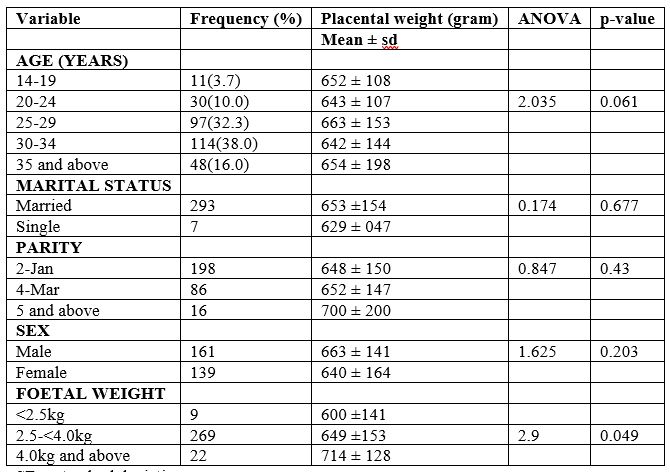
SD—standard deviation.
Table 3 showed that most placentae delivered within the period weighed between 700-800 gram 129(43.0%) followed by placental weight of 500-600 gram 121 (40.0%) and the least was placental weight of 100-200 gram 1(0.3%).
Table 3: The frequency distribution of placental weights
SD—standard deviation.
Table 3 showed that most placentae delivered within the period weighed between 700-800 gram 129(43.0%) followed by placental weight of 500-600 gram 121 (40.0%) and the least was placental weight of 100-200 gram 1(0.3%).
Table 3: The frequency distribution of placental weights

The mean birth weight of the neonate increased with a corresponding increase in placental weight as shown in fig 1. The mean birth weight increased to the maximum of 3.69kg when the placental weight was 900gram and subsequently decreased with increase in placental weight to 3.30kg when the placental weight was 1,200gram.
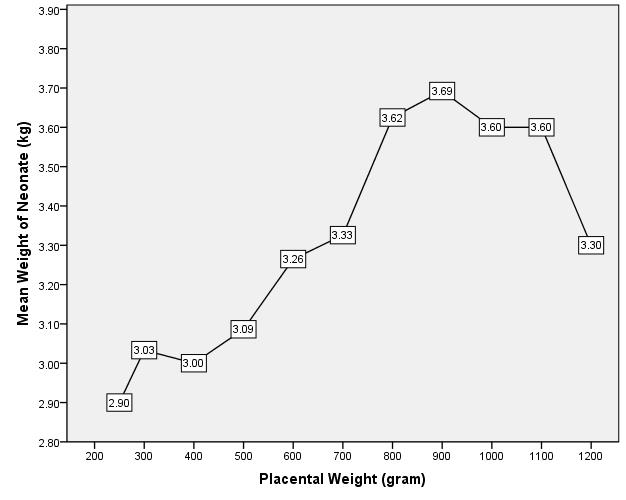
Figure 1: The relationship between the weights of placenta and the mean birth weights of the neonates
Table 4 showed the correlation between placental weight and foetal outcome. When the placental weight was compared with birth weight using the Pearson’s correlation, there was significant positive correlation between placental weight and birth weight (r = 0.330; p value = 0.000). There was also significant positive correlation between placental weight and length of the neonate (r = 0.208; p-value = 0.000).
Table 4: Correlation between placental weight and foetal outcome.
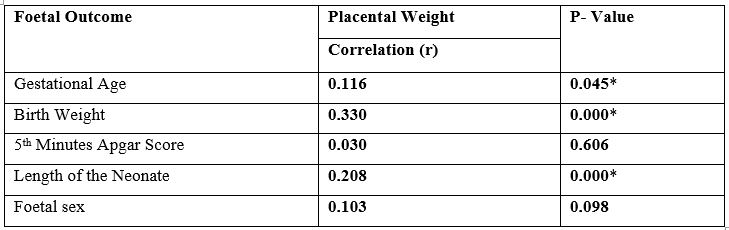
*Correlation is significant at less than 0.05 level (2-tailed)
Discussion
Studies pertaining to placental weight at term showed that placental weight varies from geographical location [3,4,10]. In this present study the mean placental weight was 652 ±152g and ranged from 250g to 1,200g. The mean placental weight is comparable to 657g and 643g reported in Western Nigeria and Europe respectively [3,6]. However, it is higher than 580g, 587 and 470 g reported in Nigeria, Asia and Ukraine respectively [11-13]. The variations observed in the mean placental weight may be due variation in control mechanisms for the growth of placenta such as ethnicity, genetic factors, nutrition, variations in placental weighing and the cord clamping time [1,13]. This showed that human neonates exhibit wider variations in terms of the weight of their placenta and results of these studies supported this finding.
The mean birth weight observed in this study was 3.309 ±0.522kg and ranged between 2.0kg to 5.9kg. This finding is similar to study in Northern Nigeria [10]. The mean birth weight of the neonate in this study is lower than 3.42kg and 3.40kg reported in Ukraine and Nigeria respectively; but higher than 3.275kg and 3.103kg in Northern Nigeria and Caribbean region respectively [1,12-14]. These observed differences in mean birth weights of neonates may be due to altitude; race, maternal nutrition, and maternal medical condition [3,10].
The age range of the participants in this study was 14-41years with a mean of 29.60 ±4.845years and was similar to subject in previous studies [10,15]. The mean gestational age in the study (38.94 ±1.326weeks) is similar to a study in Northern Nigeria and Italy [1,16], but lower than 39.9weeks reported in western Europe [17]. There was an increase in placental weight with increase in parity of the participants in the study. This finding is in agreement with a study in Nigeria which showed that placentae and babies from multiparous women at term were heavier than those from primiparous women [1].
In this present study the placental weight increased with increase in birth weight and was positively correlated with birth weight. This means that foetuses with large placenta were likely to have normal birth weight or to be macrosomic. The positive correlation between the placental weight and new born weight noted in the study were also, observed by previous authors [3,18]. Staribratova et al reported a significant positive correlation between placental weight and foetal weight [19]. A study in Asia between placental weight and obstetric outcome also, found a statistically significant relationship between placental weight and birth weight [20].
Concerning the relationship between placental weight and length of the neonate, there was a significant positive correlation. This is comparable to the study by Yu-Fang Lo et al in Taiwan which noted a positive correlation between body length of neonate and placental weight and suggested that growth of the placenta and body length may be under similar control mechanisms some of which are likely to be genetic in origin [20].
The weight of the placenta which correlated positively with the weight of the baby and length of baby in this study showed that functional placenta is necessary for optimal growth and development of the foetus. This strong relationship between the placenta and the foetus suggests that the wellbeing of the foetus is highly dependent on the placenta since it serves as a link between the mother and the developing foetus for nutritional support, excretory functions as well as immunological and hormonal support. Large placental size provides a large surface area for the exchange of substances from the mother to the foetus resulting in high foetal weight. It then implies that, factors which directly affect the weight of the placenta will indirectly affect the weight and length of the foetus. Such factors may possibly include nutrition, maternal anaemia, altitude, hypertension, maternal diabetes mellitus and other chronic medical illness [3].
This study also observed that foetal weight declined with the placental weight above 900g. The reason for the finding may be that the function of the placenta is impaired with very large placenta. Past studies indicated that high placenta weight was associated with a poor perinatal outcome, a low Apgar score, respiratory distress syndrome and perinatal death [1]. Also, disproportionately large placenta could reflect an acute placental injury resulting in villous oedema or a chronic process requiring placental overgrowth, such as maternal anaemia, impaired glucose tolerance and malnutrition [1,21]. Infants with such abnormal ratios are at increased risk of perinatal death and intrauterine growth restriction [22].
Conclusion and Recommendation
The present study showed that the placental weight is variable; however, most cases had normal placenta. There was a significant positive correlation between the placental weight and birth weight and length of the neonate. As part of routine postnatal examination, there should be appropriate examination and documentation of placental weights and abnormalities as this will provide more information on foetal wellbeing and neonatal outcome. Since placental size correlated positively with foetal weight and foetal length, future exploration of antenatal measurement of placental size for assessment of foetal growth and well-being should be explored.
Financial support and sponsorship: Nil.
Conflicts of Interest: There are no conflicts of interest.
References
- Panti AA, Ekele BA, Nwobodo EI, Yakubu A. The relationship between the weight of the placenta and birth weight of the neonate in a Nigerian Hospital. Niger Med J 2012; 53: 80-84
- Agboola A. The placenta, umbilical cord and membranes. In: Textbookof Obstetrics and Gynecology for Medical Students. 2nd ed. Ibadan,Nigeria: Heinemann Educational Books; 2006; p. 265-273.
- Nwogu CM, Adetuyi IE, Okunade KS, Osanyin GE, Oluwole AA. Placental weight and perinatal outcome among parturients at a university teaching hospital in Lagos, Nigeria. Trop J Obstet Gynaecol 2018; 35: 322-326.
- Asgharnia M, Esmailpour N, Poorghorban M, Atrkar‑Roshan Z.Placental weight and its association with maternal and neonatal characteristics. Acta Med Iran 2008; 46: 467‑472.
- Agboola A. pregnancy induced hypertension pre-eclampsia and chronic hypertension. In: Textbookof Obstetrics and Gynecology for Medical Students. 2nd ed. Ibadan,Nigeria: Heinemann Educational Books; 2006; p. 348-355.
- Barker DJ, Bull AR, Osmond C, Simmonds SJ. Foetal and placental size and risk of hypertension in adult life. BMJ. 1990; 301: 259–262.
- Robertson CM, Svenson LW, Kyle JM. Birth weight by gestational age for Alberta liveborn infants, 1985 through 1998. J Obstet Gynaecol Can. 2002; 24: 138-48.
- Roland MC, Friis CM, Voldner N, Godang K, Bollerslev J, Haugen G, et al. Foetal growth versus birthweight: The role of placenta versus otherdeterminants. PLoS One 2012; 7: e39324.
- Leary SD, Godfrey KM, Greenaway LJ, Davill VA, Fall CH. Contribution of the umbilical cord and membranes to untrimmed placental weight. Placenta. 2003; 24: 276–278.
- Onankpa BO, Airede KI, Ahmed H, Jiya NM. The birth weight of apparently healthy Nigerian newborn in Sokoto. Sahel Med J. 2006; 1: 19–22.
- Adesina KT, Ogunlaja OO, Aboyeji AP, Akande HJ, Adeniran AS, Olarinoye A, et al. Relationship between gross placental characteristics and perinatal outcome of low-risk singleton deliveries. Niger Postgrad Med J 2016; 23: 191-5.
- Patimah S, Yasmin Syauqi Y, Thaha AR. The Correlation between Placental Weight and Birth Weight. International Proceedings of Chemical, Biological and Environmental Engineering, 2015; 86: 58-64
- Little RE, Zadorozhnaja TD, Hulchiy OP, Mendel NA, Shkyryak-Nyzhnyk ZA, Chyslovska N, et al. Placental weight and its ratio to birthweight in a Ukrainian city. Early Hum Dev. 2003; 71: 117–27.
- Lurie S, Feinstein M, Mamet Y. Human foetal-placental weight ratio in normal singleton near-term pregnancies. Gynecol Obstet Invest. 1999; 48: 155–7.
- Adebami OJ, Owa JA, Oyedeji GA. Factor associated with placenta weight and placental weight/birtweight percent (placental ratio) among mothers in Ilesa, Southwesttern Nigeria. Int J Trop Med. 2007; 2(2):68–73.
- Burkhardt T, Schaffer L, Schneider C, Zimmermann R, Kurmanavicius J. Reference values for the weight of freshly delivered term placentas and for placental weight-birth weight ratios. Euro J Obstet Gynecol Reprod Biol. 2006; 128: 248–252.
- Janthanaphan M, Kor-Anantakul O, Geater A. Placental weight and its ratio to birth weight in normal pregnancy at Songkhlanagarind Hospital. J Med Assoc Thai. 2006; 89: 130–137.
- Madkar C, Musale J, Deshpande H, Shitole R. A study of placental weight and birth weight ratio (Pw/Bw) and its effects on perinatal outcome.Indian J Obstet Gynaecol 2015; 2: 1‑6.
- Staribratova D, Milchev N. Placental parameters and foetal outcome. Akush Ginekol (Sofiia) 2009; 48: 37-40.
- Lo YF, Jeng MJ, Lee YS, Soong WJ, Hwang B. Placental weight and birth characteristics of healthy singleton newborns. Acta Paediatr Taiwan 2002; 43: 21-25.
- Lao TT, Tam KF. Placental ratio and anaemia in third-trimester pregnancy. J Reprod Med. 2000; 45: 923–928.
- Wallace JM, Horgan GW, Bhattacharya S. Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in women delivering singleton babies. Placenta 2012; 33: 611-618.

