Immune Stimulation and Proapoptotic Effect of Honey Bee Propolis Against Solid Ehrlich Carcinoma Bearing Mice
Nermeen M El Bakary*
Radiation Biology Department, National Centre for Radiation Research and Technology, Egypt
Received Date: 25/03/2021; Published Date: 08/04/2021
*Corresponding author: Mohin IJ, Radiation Biology Department, National Centre for Radiation Research and Technology, Egyptian Atomic Energy authority, Cairo, Egypt
Abstract
The increased interest in new approaches to the immunotherapy of cancer, and a considerable demand for therapeutic agents which can modulate the several forms of immunodeficiency have encouraged studies on the immunomodulatory mechanism of natural and synthetic substances. The present work was an endeavor to evaluate the imuno stimulating and pro apoptotic effect of Propolis on the tumor growth targeting the improvement of cancer therapeutic protocols. Propolis (100 mg/kg body weight/day) was injected intraperitoneal to mice bearing 1cm3solid tumor of Ehrlich Ascites Carcinoma (EAC) for 21 consecutive days. Treatment with Propolis markedly suppresses the proliferation of tumor in EAC mice. The activity of IFN-γ was significantly decreased compared to EAC mice. The concentrations of m-RNA for angiogenic factors (TNF-α), free radicals as well as Nitric Oxide (NO) concentration were significantly decreased collimated with improvements in apoptotic regulators (Caspase-3 activity) compared to EAC mice. Moreover, the histopathological investigation confirms the improvement exerted by Propolis even in EAC mice group. Hence, Propolis might represent a potential therapeutic strategy for solid tumor treatment.
Keywords: Propolis; TNF-α; IFNγ; Apoptosis; Caspase-3; Histopathology
Introduction
Cancer is considered one of the most common causes of morbidity and mortality worldwide. There are 3 major approaches to treat cancer, that is, surgical excision, irradiation, and chemotherapy. The comparative value of these approaches depends on tumor type and stage of cancer. The major therapeutic approach for the treatment of benign and metastasized cancer is chemotherapy; however, this treatment suffers from several limitations including (1) most chemotherapeutic drugs lack selectivity toward cancer cells and hence result in severe toxicity and side effects and (2) P-glycoproteins in the cancer cells activate and mediate multidrug resistance in malignant cells (Ishida et al., 2018) [1]. Heterogeneous cell populations in individual cancers or different tumors also give rise to a variety of drug-resistant cancer stem cellsthat contribute to tumor relapse. Zimmerman et al. (2014) [2] have described the limited aqueous solubility of plant-derived anticancer drugs as a hurdle to their effective use. These are often hydrophobic in nature and require different solvents to formulate the dosage that also generate severe toxicity. Hence, it is extremely important to design NEW (natural, efficient, and welfare) drugs with additional useful characteristics including cost-effectiveness and targeted delivery. Discovery of NEW selectively targeting drugs is still slow and has high failure rate, particularly in the advanced stages of cancer.
The target of much research has been on the discovery of natural and synthetic compounds that can be used in the prevention and/or treatment of cancer. Natural products of either plant or animal origin that exhibited antitumor activity has been discovered (Pezzuto, 1997) [3]. The increased interest in new approaches to the immunotherapy of cancer, and a considerable demand for therapeutic agents which can modulate the several forms of immunodeficiency have encouraged studies on the immunomodulatory mechanism of natural and synthetic substances (Mirandola et al., 2002) [4]. Propolis (bee glue) is the generic name for the resinous substance collected by honey bees from various plant sources and used by bees to seal holes in their honeycombs, smooth out the internal walls, and protect the entrance against intruders. It is rich in biochemical constituents, including mostly a mixture of polyphenols, flavonoid aglycones, phenolic acid and their esters, and phenolic aldehydes and ketones, terpenes, sterols, vitamins, amino acids, etc. It was also demonstrated that propolis and some of its active substances have a pronounced cytostatic, anticarcinogenic and antitumour effect both in “in vitro” and “in vivo” tumor models (Attia et al., 2007) [5]. It has been suggested that the therapeutic activities of propolis depend mainly on the presence of flavonoids. Flavonoids have also been reported to induce the immune system, and to act as strong oxygen radical scavengers (Wleklik et al., 1997; Orsˇolic´ et al., 2003 & Azab et al., 2020) [6-8].
This led us to compare how the administration of polyphenolic compound like propolis influence on Ehrlich ascites tumor growth. Thus, we analyzed not only tumor growth but also parameters of immunomodulatory response of Ehrlich solid tumor bearing mice.
Materials and Methods
Chemicals Propolis, and other chemicals and reagent in this study were obtained from Sigma-Aldrich Chemical Co., (Gillingham, UK). Propolis was dissolved in ethanol and injected i.p. at a dose of 100 mg/kg body weight for 21 consecutive days.
Tumor transplantation:
In the present study Cell line of Ehrlich Ascites Carcinoma (EAC) was used as a model of solid carcinoma by inoculation in the right thigh of albino mice. The parent line was supplied as gift from Egyptian National Cancer Institute (NCI), Cairo University. Human breast cancer is the source of EAC cells upon modified to grow in female Swiss albino mice. The cell line of EAC was maintained by intraperitoneal injection (i.p.) of 2.5 million cells per animal. Bright line haemocytometer was used to count the EAC before i.p. injection and the dilution was done using physiological sterile saline solution. In order to develop Ehrlich solid tumor (EST) in thigh, 0.2 ml EAC cells (2.5x106 cells /mouse) were inoculated subcutaneously (s.c.) in the right thigh of the lower limb of female mouse (Medhat et al., 2017; Hafez et al., 2020) [9,10].
Animals Categorize:
In this study, we used 45 mice weighing about 25 g. All the experiments were conducted under national research centre guidelines for the use and care for laboratory animals and were approved by an independent ethics committee of the National Center for Radiation Research and Technology (NCRRT).
The animals were categorized into 3 equal groups of 15 mice each as follows:
Group (1): Control (C): Mice neither treated nor irradiated.
Group (2): (EAC): Mice bearing solid Ehrlich tumor.
Group (3): (EAC+P): Mice bearing solid Ehrlich tumor were injected i.p. with proplis for 21 consecutive days starting at the 11 days after EAC inoculation.
Tumor volume monitoring:
Tumor volume was measured at different time intervals during the experimental period using a vernier caliper on the 7th,15th, 21th days from the tumor has been reached 1cm3 during the experimental period. The volume of solid tumor was calculated using formula [A * B2 * 0:52], where A and B are the longest and the shortest diameter of tumor, respectively (Papadopoulos et al., 1989) [11].
Mice were sacrificed at the end of experiment. The skeletal muscle (normal control), tumor tissues and liver were collected for biochemical investigations.
Quantitative real-time PCR:
RNA isolation and reverse transcription: RNA was extracted from the tumor tissue homogenate using the RNeasy plus mini kit (Qiagen, Venlo, The Netherlands), according to the manufacturer’s instructions. Genomic DNA was eliminated by a DNase-on-column treatment supplied with the kit. The RNA concentration was determining spectrophotometrically at 260 nm using the Nano Drop ND-1000 spectrophotometer (Thermo Fisher scientific, Waltham, USA) and RNA purity was checked by means of the absorbance ratio at 260/280 nm. RNA integrity was assessed by electrophoresis on 2% agarose gels. RNA (1 μg) was used in the subsequent cDNA synthesis reaction, which was performed using the Reverse Transcription System (Promega, Leiden, The Netherlands). Total RNA was incubated at 70°C for 10 min to prevent secondary structures. The RNA was supplemented with MgCl2 (25mM), RTase buffer (10X), dNTP mixture (10mM), oligod (t) primers, RNase inhibitor (20 U) and AMV reverse transcriptase (20 U/μl). This mixture was incubated at 42°C for 1 h.
Quantitative real time PCR: qRT-PCR was performed in an optical 96-well plate with an ABI PRISM 7500 fast sequence detection system (Applied Biosystems, Carlsbad, California) and universal cycling conditions of 40 cycles of 15 s at 95°C and 60 s at 60°C after an initial denaturation step at 95°C for 10 min. Each 10 μl reaction contained 5 μl SYBR Green Master Mix (Applied Biosystems), 0.3 μl gene-specific forward and reverses primers (10 μM), 2.5 μl cDNA and 1.9 μl nuclease-free water. The sequences of PCR primer pairs used for each gene are shown in Table 1. Data were analysed with the ABI Prism sequence detection system software and quantified using the v1·7 Sequence Detection Software from PE Biosystems (Foster City, CA). Relative expression of studied genes was calculated using the comparative threshold cycle method. All values were normalized to the endogenous control GAPDH (Livak and Schmittgen 2001) [12].
Table 1: Primers used for QRT-PCR.

ELISA detection:
Enzyme-linked immune sorbent assay (ELISA) for levels of IFN-γ and Caspase-3 were determined by using ELISA Kit (R& D systems) according to the manufacturer’s instructions on the supernatants of sample tissue homogenates. In brief, microplates were coated with 100 μl/well of capture antibody, and then they were incubated overnight at 4°C. After washes, the plates were blocked with assay diluent at room temperature (RT) for 1 h. One hundred microliters of a serum sample were added to each well of the plate, followed by incubation for 2 h at RT. Working detector was added into each well, and the plate was incubated for an additional 1h at RT before the addition of substrate solution. The reaction was stopped by adding stop solution. The absorbance was read using ELISA reader. The concentrations were calculated from standard curve according to the instructions in the protocol.
Nitric oxide determination
Nitric oxide (NO) level in the livert issues was determined colormetric as nitrite by Griess reaction (Miranda et al., 2001) [13].
Measurement of radical-scavenging ability in hepatic tissue using electron spin resonance spectroscopy
The liver tissues were quickly removed from mice and were gently lyophilized and were evaporated to dryness under vacuum. All samples were dissolved in a small volume of toluene, and were transferred to a round ESR cell. The cells were capped with a rubber septum and were thoroughly deoxygenated by nitrogen bubbling before ESR spectroscopy was performed. The ESR spectra were recorded at room temperature using a Bruker EPR ER-200D spectrometer, and spectral accumulation was done by using a Bruker ER-140(ASPECT 2000) data systems. The microwave power was 2 mW, the modulation amplitude was 1 G and 1 E4 receiver gain. The response time constant was 10 ms, with a field-sweeping rate of 100 G/ 42 s. The height of powder sample inside the quartz tube was about 10 mm. ESR spectral analyses were performed through the use of a computer simulation program (Oehle and Janzen, 1982) [14].
Histopathological study:
Autopsy samples were taken from the thigh muscle of mice in different groups and fixed in 10% formol saline for twenty-four hours. Washing was done in tap water then serial dilutions of alcohol (methyl, ethyl and absolute ethyl) were used for dehydration. Specimens were cleared in xylene and embedded in paraffin at 56 degree in hot air oven for twenty-four hours. Paraffin bees wax tissue blocks were prepare d for sectioning at 4 microns thickness by slidge microtome. The obtained tissue sections were collected on glass slides, deparaffinized, and stained by hematoxylin and eosin stain for routine examination through the light electric Microscope (Banchroft et al. 1996).
Statistics:
Statistical analysis was performed by one way analysis of variance (ANOVA) followed by Duncan’s Multiple Range test by using statistical package of social science (SPSS) version 20.0 for windows. P ≤ 0.05 were considered as a level of significance.
Result:
Impact of Propolis on tumor volume in different mice groups.
Table (2) demonstrated that the size of solid EAC tumor reached 6500.40 mm3 at the 7th days from the tumor has been reached 1cm3 during the experimental period, and enlarged to 38700.46 mm3 at the end of the experiment. A gradual significant decrease in the tumor size during treatment of EAC-bearing mice with P was demonstrated, compared to untreated EAC-bearing mice.
Table 2: Statistical significance of the tumor volume in different groups.
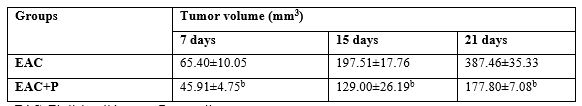
EAC: Ehrlich solid tumor, P: propolis
Each value represents the mean ± SE (n=6).
b: significantly different from EAC.
Impact of P administration to mice on angiogenic Parameters
Treatment of mice bearing solid tumor with P (E+ p group) induced significant decreases (P<0.05) in TNF-α concentration. Also, the NO contents of tumor tissue were significantly ameliorated. (Table 3).
Table 3: mRNA expression of tumor necrosis factor alpha (TNF-α) and No concentration in the right thigh muscle (control) or tumor tissue in different groups.

C: control, EAC: Ehrlich solid tumor, P: propolis
Each value represents the mean ± SE (n=6).
a: significantly different from control. b: significantly different from EAC.
Impact of P administration to mice on Caspase-3, IFN-γ
Table (4) showed that mice bearing solid EAC tumors manifested that IFN-γ and caspase-3 levels were significantly decreased respectively, compared to non EAC-bearing mice. Treatment of EAC-bearing mice with P resulted in a pronounced elevation in IFN-γ and caspase-3 respectively, compared to untreated EAC-bearing mice.
Table 4: Interferon gamma concentration (IFN-γ), caspase-3 concentration in the right thigh muscle or tumor tissue in different groups.

C: control, EAC: Ehrlich solid tumor, P: propolis
Each value represents the mean ± SE (n=6).
a: significantly different from control. b: significantly different from EAC.
The effect of liver tissue ESR free radical in different groups
Table (5) showed that mice bearing solid EAC tumors manifested high significant increase for the free radical peak intensity, compared to non EAC-bearing mice. Treatment of EAC-bearing mice with P resulted in a pronounced decline in free radical peak intensity, compared to untreated EAC-bearing mice.
Table 5: Statistical Analysis for the peak intensity for liver tissue ESR free radical in different groups
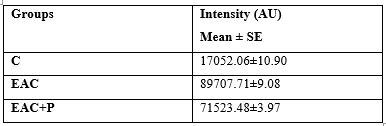
C: control, EAC: Ehrlich solid tumor, P: propolis
Each value represents the mean ± SE (n=6).
Impact of P treatment on histopathology of EAC bearing mice
Photomicrographic examinations of control mice thigh muscles tissue demonstrates normal histological structure of striated bundles (Figure 1A). However, photomicrograph of EAC mice displayed compactness of the tumor cells scattered within the muscular tissues and aggregated in focal manner infiltrating and penetrating the muscle bundles. Groups of large round and polygonal cells with pleomorphic shapes, hyperchromatic nuclei and binucleation, several degrees of cellular and nuclear pleomorphism were observed (Figure 1B)). On the other hand, the histopathological investigation performed showed a great destruction of tumor tissue represented by the appearance of dead and necrotic cells after P administration.
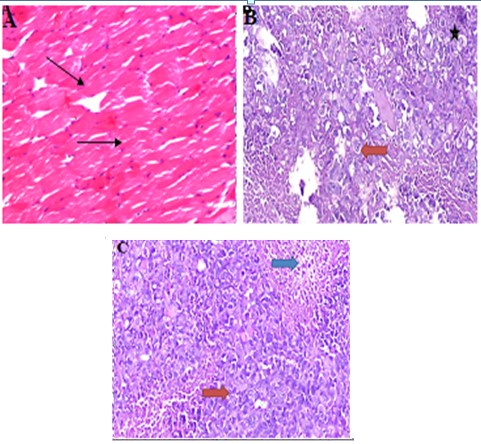
Figure 1: Photomicrographs sections of right thigh muscles. A: Normal control; C displayed normal histological structure of striated bundles. (→). B: Mice bearing solid tumor; E Ehrlich tumor cells exhibiting pleomorphism, hyperchromatisa (*), infiltrating and penetrating the muscle bundles and aggregated in focal manner in between (→).C: Propolis showed malignant cells (red arrow) surrounded by 10% of tumor necrosis (blue arrow).
Discussion
It is expected in this century that cancer will be the most important reason of death and the leading obstacle to rise in life expectancy; over 18 million new cancer cases with over 50 % mortality has been mentioned in 2018 estimates. Due to the great importance of the immune system in the development of cancer, there has been an attempt to develop immunotherapies directed against tumors with the aim of increasing the antitumor immune response and consequently the eradication of the neoplastic in progress (Hafez et al., 2020; Celińska-Janowicz et al., 2018) [8,15].
In an attempt to improve cancer therapeutic protocols, this study was undertaken to evaluate the antitumor effect of Propolis against solid EC tumors in female mice. Different molecular targets were analyzed in order to explore the immune modulation and the suppression effect of Propolis on the growth of solid tumor.
In this study, a significant upregulation was recorded in IFN-γ activity and TNF-α messenger RNA (mRNA) expression in solid EC tumors, compared to non EC-bearing mice.). Its potential as a protumor factor also has a long history (Zaidi and Merlino 2011) [16]. One common theme that seems to emerge from the studies that show protumorigenic effects of IFNG is that tumors that are exposed to IFNG show greater immunoevasive capabilities. For example, several IFNG pathway target genes are known to be involved in immunosuppressive and immunoevasive mechanisms that are geared toward suppression of CTL- and NK cell-mediated antitumor immune responses (RazaZaid,2019) [17]. TNF-α is a major inflammatory mediator that induces multiple changes in endothelial cell gene expression, including induction of adhesion molecules, integrins, and MMPs, therefore acting as an autocrine growth factor for tumor angiogenesis (Song et al., 2012, El- Bakary et al., 2020) [18,8]. Further, the significant increase (P‹0.05) in NO concentration of EAC bearing mice might be due to TNF-α over expression (El- Bakary et al., 2020) [8]. It has been demonstrated that TNF-α is a mediator of NO synthesis (Liu et al., 2010) [19]. NO is produced from L-arginine by a family of NO synthase (NOS) isoenzymes. These enzymes comprise three distinct isoforms, encoded by three different genes and include neuronal (nNOS codified by NOS-1), inducible (iNOS/NOS-2), and endothelial (eNOS/NOS-3) forms. Although, at baseline, the main source of plasma NO is related to eNOS, during several clinical conditions, such as inflammation, iNOS is activated (Assmann et al., 2016) [20]. The inducible form of nitric oxide synthase is expressed mainly through TNF-α activated pathway (El Bakary et al., 2020; Medhat et al., 2017) [8,9]. The disturbance in the angiogenic and apoptotic regulators leads to tumor proliferation and growth, which was clearly demonstrated by the increase in EC tumor volume. Neovascularization enhances the ability of the tumor to grow and increases its invasiveness and metastatic ability (Dhankhar et al., 2010; El Bakary et al., 2020) [8,21]. Apoptosis is a programmed cell death that maintains the stability of the internal environment through removing genetic mutations and unstable cells. However, this process is inhibited in cancer which leads to the accumulation of various genetically unstable cells. Our results demonstrated a significant decline in the level of apoptotic molecule (caspase-3) in the solid EC tumors, compared to non-EC-bearing mice. Caspase-3 mediated apoptosis is a major focus in the field of cancer growth inhibition, because activation of proteolytic caspase cascade is a critical component in the execution in apoptotic cell death (Azab et al., 2020; Choi and Kim, 2009) [8,22]. The increase in TNF-α expression is accompanied with decrease in caspase -3 activities. These findings are online with (Zhang et al., 2018) [23] who found that Knockdown of trans membrane TNF-α expression enhances the therapeutic efficacy of Doxrubicin in a xenograft mouse model where the combination of tmTNF-α inhibition and DOX treatment resulted in much more pronounced suppression of tumor growth The current data revealed that the upregulation of TNF-α genes and induction of IFN-γ activity and No concentration along with apoptosis suppression in solid EC-bearing mice collectively enhance tumor cell proliferation and neovascularization, which ultimately resulted in the acceleration of tumor growth, invasiveness, and metastatic ability of tumor cells.
The current modalities of cancer treatment are mainly comprised of surgery, radiation-based therapy, chemotherapy, gene therapy and/or hormonal therapy, another approach that has gained importance is the use of bio toxins or api-toxin such as animal venoms as cancer therapeutic agents. These bio toxins are produced by living organisms as a defense mechanism against predators and are known to have both toxicological as well as pharmacological effects (El Bakary et al., 2020; Zhang, 2015) [8, 24].
The result of this study revealed that treatment of EC-bearing mice with P significantly reduced the growth of solid tumors. As a key player in tumor proliferation, angiogenesis, and inflammatory cascade induction, the down regulation of TNF-α expression following treatment of irradiated and non-irradiated EC-bearing mice with P reduced tumor growth via multiple mechanisms, including suppression of tumor cell proliferation (reduction in tumor volume), angiogenesis (down regulation of tumor TNF-α expression and NO concentration), and enhancing apoptosis (induction of tumor caspase-3). Propolis evokes several therapeutic properties, including anticancer activity. These activities are attributed to the action of polyphenols. Previously it has been demonstrated, that one of the most abundant polyphenolic compounds in ethanolic extracts of propolis are chrysin (Celińska-Janowicz et al.,2018) [15]. propolis had good antitumor effects on different cancer cells such as the human breast cancer cells (MCF-7 and MDA-MB-231), lung cancer cells (A549), human cervical carcinoma cell (HeLa). And the antitumor bioactive constituents are flavonoids and esters. In addition, propolis exhibit excellent anti-inflammatory activities in macrophages (Raw 264.7), ox-LDL stimulated HUVECs and intestinal epithelial Caco-2 cells by modulating key inflammatory mediators of mRNA transcription, inhibiting the production of specific inflammatory cytokines, blocking the activation of nuclear factor NF-κB, tumor necrosis factor alpha, and activating AMPK and ERK signaling pathway (Chang et al., 2017) [25].
Where the earliest stages of angiogenesis are defined by vasodilatation mediated by NO and an increased vascular permeability of pre-existing capillaries or post-capillary venules in response to overexpressed VEGF (Azab et al., 2020; Kumar and Kuttan, 2011) [8,16], and the inducible form of nitric oxide synthase is expressed mainly through TNF-α activated pathway (Azab et al., 2020) [8], So TNF-α is the maestro for controlling angiogenesis process. P has demonstrated potential efficacy in inhibiting TNF-α and No concentration (Chang et al., 2017) [26]. NO, a physiological signaling molecule, is involved in many cellular functions, including cell proliferation, survival and death. A recent study suggested that LPS/TLR4-induced signaling cascades leads to inducible nitric oxide synthase (iNOS) induction, and inhibition of iNOS might be as a novel effective target therapy against triple negative breast cancer. It was found that Chinese propolis and CAPE obviously inhibited the production of NO, which might inhibit MDA-MB-231 cells survival (Granados-Principal et al., 2015) [27].
Apoptosis was another major cause for Chinese propolis and CAPE to inhibit LPS-stimulated MDA-MB-231 cells survival. It was reported that propolis could induce cancer cells apoptosis. It was also found that Chinese propolis and CAPE activated caspase 3- the executor of apoptosis in LPS-stimulated breast cancer cells, which might be induced by activating autophagy and depressing TLR4 signaling pathway (Frozza et al., 2014) [28].
In conclusion, treatment of E, E+R groups with P or Ch exerted a marked effect in the retardation of tumor growth as compared to tumor bearing mice group. These observations could be attributed to the immune stimulation, antiappoptotic and antioxidant capacity of P or Ch.
References
- Ishida Y, Gao R, Shah N et al. Anticancer Activity in Honeybee Propolis: Functional Insights to the Role of Caffeic Acid Phenethyl Ester and Its Complex With γ-Cyclodextrin. Integr Cancer Ther. 2018; 17(3): 867-873.
- Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev. 2014; 40: 716-722.
- Pezzuto JM. Plant derived anticancer agents. Biochem. Pharmacol., 1997; 53: 121.
- Mirandola L, Justo GZ, Queiroz MLS. Modulation by Acanthospermum austral extracts of the tumor induced haematopoietic changes in mice. Immunopharmacol. Immunotoxicol., 2002; 24: 275-288.
- WaelY Attia, Kamal A El-Shaikh, Mohamed S Gabry Gehan A. Othman PROPHYLACTIC EFFECT OF PROPOLIS AGAINST TUMOR GROWTH IN MICE THROUGH STIMULATION OF THE IMMUNE SYSTEM, Egypt. J. Exp. Biol. (Zool.), 2007; 3: 219-228.
- Wleklik M, Zahorska R, Luczak V. Acta Microbiol. Pol., 1997; 32, 1141-1148.
- Orsˇolic´ N., Basˇic´ I., Mellifera, 2003; 3, 56-64.
- Azab KS, El Fatih NM, El Tawill Gh, El Bakary NM. Pro-apoptotic and anti-neoplastic impact of luteolin on solid Ehrlich carcinoma.bearing mice exposed to gamma radiation. J Cancer Res Ther. 2020; 16(6): 1506-1516.
- Medhat AM, Azab Kh Sh, Said MM,El Fatih NM, El Bakary NM (2017): Antitumor and radiosensitizing synergistic effects of apigenin and cryptotanshinone against solid Ehrlich carcinoma in female mice, Tumor Biology , 1-13.
- El Bakary NM, Alsharkawy AZ, Shouaib ZA, Barakat EMS. Role of Bee Venom and Melittin on Restraining Angiogenesis and Metastasis in γ-Irradiated Solid Ehrlich Carcinoma-Bearing Mice. Integr Cancer Ther. 2020; 19: 1534735420944476
- Papadopoulos D, Kimler BF, Estes NC, et al. Growth delay effect of combined interstitial hyperthermia and branchytherapy in a rat solid tumor model. Anticancer Res 1989; 9:45-47.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001; 25: 402–408.
- Miranda KM, Espey MG, Wink DA. A rapid simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide.2001; 5(1): 62-71.
- Oehler, U. M.; Janzen, E. G. Simulation of isotropic electron spin resonance spectra: a transportable basic program. Can. J. Chem. 1982; 60: 1542-1548.
- Celińska-Janowicz K1, Zaręba I2, Lazarek U1, Teul J1, Tomczyk M3, Pałka J2, et al. Constituents of Propolis: Chrysin, Caffeic Acid, p-Coumaric Acid, and Ferulic Acid Induce PRODH/POX-Dependent Apoptosis in Human Tongue Squamous Cell Carcinoma Cell (CAL-27). 2018; 9: 336.
- Kumar, P. P. and Kuttan, G. Nomilin inhibits tumor-specific angiogenesis by downregulating VEGF, NO and proinflammatory cytokine profile and also by inhibiting the activation of MMP-2 and MMP-9. European Journal of Pharmacology, 2011; 668: 450-458.
- RazaZaid M. The Interferon-Gamma Paradox in Cancer journal of Interferon & Cytokine ResearchVol. 39, No. 1 Research ReportsFree Access, 2019.
- Song K, Zhu F, Zhang HZ, et al. Tumor necrosis factor-α enhanced fusions between oral squamous cell carcinoma cells and endothelial cells via VCAM-1/VLA-4 pathway. Exp Cell Res 2012; 318: 1707–1715.
- Liu, J. G.; Zhao, H. J.; Yan-Juan, L. and Wang, X. L. Effect of selenium-enriched malt on VEGF and several relevant angiogenic cytokines in diethylnitrosamine-induced hepato carcinoma rats. Journal of Trace Elements in Medicine and Biology, 2010; 24: 52-57.
- Assmann TS, Brondani LA, Bouças AP, Rheinheimer J, Souza BM, Canani LH, et al. Nitric oxide levels in patients with diabetes mellitus: A systematic review and meta-analysis. Nitric Oxide 2016; 61: 1-9.
- Dhankhar R, Dahiya K, Singh V, Sangwan L, Kaushal V. Nitric oxide and cancer. J. Clin. Diag. Res. 2010; 4: 2550-2559.
- Choi EJ and Kim GH. Apigenin induces apoptosis through a mitochondria/caspase-pathway in human breast cancer MDA-MB-453 cells. J. Clin. Biochem. Nutr. 2009; 44: 260-265.
- Zhang Z, LinG, Yan Y, et al. Transmembrane TNF-alpha promotes chemoresistance in breast cancer cells, Oncogene.; 2018; 37(25): 3456–3470.
- Zhang Y. Why do we study animal toxins? Dongwuxue Yanjiu. 2015; 36:183–222. [PubMed: 26228472]
- H Chang, Wang Y, Yin X et al. Ethanol extract of propolis and its constituent caffeic acid phenethyl ester inhibit breast cancer cells proliferation in inflammatory microenvironment by inhibiting TLR4 signal pathway and inducing apoptosis and autophagy, BMC Complementary and Alternative Medicine 2017; 17:471.
- Meng X1, Fang S1, Zhang Z1, Wang Y1, You C1, Zhang J1, Yan H1. Preventive effect of chrysin on experimental autoimmune uveitis triggered by injection of human IRBP peptide 1-20 in mice. Cell Mol Immunol. 2017; 14(8): 702-711.
- Granados-Principal S, Liu Y, Guevara ML, Blanco E, Choi DS, Qian W, Patel T, Rodriguez AA, Cusimano J, Weiss HL. Inhibition of iNOS as a novel effective targeted therapy against triple-negative breast cancer. Breast Cancer Res. 2015; 17: 25.
- Frozza CO, Ribeiro Tda S, Gambato G, Menti C, Moura S, Pinto PM, Staats CC, Padilha FF, Begnini KR, de Leon PM. Proteomic analysis identifies differentially expressed proteins after red propolis treatment in Hep-2 cells. Food Chem Toxicol. 2014; 63: 195–204.

