Toxicity of Iron Oxide Nanoparticles, Silver Nanoparticles and Their Mixture on Antioxidant Enzymes and Free Radicals in Male Rats
Ahmed Ibrahim Younus1, Mokhtar Ibrahim Yousef2, Maher Abdel-Nabi Kamel3, Rakhad Alrawi*4 and Jubran Mohammed Abdulrahman5
1Ministry of Health, Iraq
2Department of Environmental Studies, Institute of Graduate Studies and Research, Alexandria University, Egypt
3Department of Biochemistry, Medical Research Institute, Alexandria University, Egypt
4Grand Canyon University and Arizona College. Arizona, USA
5Center of Research and Educational Studies, Ministry of Education, Iraq
Received Date: 11/11/2020; Published Date: 15/12/2020
*Corresponding author: Rakhad Alrawi, Grand Canyon University and Arizona College. Arizona, USA. E-mail: iraqppa@yahoo.com, ragad.rawai@googlemail.com
Abstract
In the present study Wistar male rats were used. Rats were divided into 4 equal groups, 10 rats each. Group 1 served as control, group 2 was administered orally with Fe2O3NPs (5 mg/kg BW; >50 nm), group 3 was treated intraperitoneally with AgNPs (50 mg/kg BW; >100 nm) and group 4 was administered with the mixture of Fe2O3NPs with AgNPs. Animals were treated with the doses every day for 79 days. Results showed significant (P < 0.05) decrease in the antioxidant enzymes (GPX, GST, CAT and SOD) and reduced glutathione (GSH) and total antioxidant capacity (TAC), while significant (P < 0.05) increase in thiobarbituric acid-reactive substances (TBARS) and nitric oxide (NO) in plasma and testes of rats treated with Fe2O3NPs, AgNPs and their combination compared to control group.
Keywords: Iron oxide nanoparticles; Silver nanoparticles; Antioxidant enzymes; Free radicals
Introduction
Nanoparticles (NPs) usually ranging in dimension from 1-100 nanometers (nm) have properties unique from their bulk equivalent, with the decrease in the dimensions of the materials to the atomic level, their properties change, the nanoparticles possess unique physic-chemical, optical and biological properties which can be manipulated suitably for desired applications [1]. The nanoparticles are broadly grouped into organic and inorganic nanoparticles. The latter have gained significant importance due to their ability to withstand adverse processing conditions [2]. Carbon and metallic nanomaterial's are among the most widely used types of engineered nanomaterials, nano-metals, such as nano-gold, nano-silver, nano-copper, nano-aluminum, nano-nickel, nano-iron, and other nanoparticles, have also been extensively studied [3]. Iron oxide nanoparticles (Fe2O3NPs) have shown wide biological applications inmagnetic resonance imaging (MRI), drug delivery, gene therapy, cancer treatments, In Vitro Diagnostics (IVD), and vaccine and antibody production. Although Fe2O3NPs have a variety of applications, few studies have demonstrated that exposure to Fe2O3NPs may lead to adverse effects, such as reproductive toxicity [4]. Silver nanoparticles (AgNPs) are widely used in products across industries; they are often used for their antimicrobial activity in medicine and are also often found in detergents. With this increase in consumer products containing silver nanoparticle, the potential for exposure to AgNPs, it highly mobile and easily transported into food chain, aquatic systems, few studies have demonstrated that exposure to AgNPs may lead to adverse effects, such as reproductive toxicity [5,6]. The previous studies demonstrated that there is no enough results on the reproductive toxicity induced by Fe2O3NPs and AgNPs, especially in combination. Therefore, the present study aimed to investigate the reproductive toxicity of iron oxide nanoparticles, silver nanoparticles and their combination in male rats. Nanotechnology has lead to the increased production and application of several nanostructure materials, including silver nanoparticles (AgNPs). This kind of nanoparticles has been incorporated into several products such as cosmetics, textiles, and medicines for their bactericidal effect [5,6]. Because of their antimicrobial, optical, and catalytic properties, silver nanoparticles (SNP) have gained particular interest for many commercial applications. According to the Woodrow Wilson Inventory, approximately 30% of all nanoparticles-enabled products contain nanosilver. Thus, SNP are highly commercialized and are now being used in many daily life products mainly because of their antimicrobial properties. In clear contrast to SNP widespread use, putative health effects have only just begun of being adequately addressed [7]. Very little is known about the toxicity of nano-sized silver particles, however, the size and surface area are recognized as important determinants for toxicity [8]. Significant concerns have been expressed about the potential risk of silver nanoparticles (AgNPs), due to the current and projected high exposure [9]. A few research groups have investigated the toxicity of silver nanocomposites and nanoparticles in cell lines to estimate viability and reactive oxygen species (ROS) generation [10-12].
Aim of the Study
The present study was carried out to investigate the reproductive toxicity of Fe2O3NPs and AgNPs alone or in combination in male rats throughout the measuring of antioxidant enzymes and free radicals.
Materials and Methods
Iron oxide nanoparticles (Fe2O3NPs), Nano powder >50 nm particle size (TEM) and Silver nanoparticles (AgNPs), Nano powder >100 nm particle size were purchased from Sigma-Aldrich Chemical Company, (St Louis, MO, USA). Iron oxide nanoparticles was dissolved in distilled water and orally treated at a dose of 5 mg/kg BW [13]. Silver nanoparticles was dissolved in distilled water and intraperitoneally injected at a dose of 50 mg/kg/day according to Sharma et al. [14].
Forty adult male Wistar rats weighing 160-170 g and 5-6 months of age of were used in the present study. Animals were obtained from Faculty of Medicine, Alexandria University, Alexandria, Egypt. Animals were kept on basal diet and tap water which were provided ad libitum. Rats were fed pellets consisted of 30% berseem (Trifolium alexandrinum) hay, 25% yellow corn, 26.2% wheat bran, 14% whole soybean meal, 3% molasses, 1% CaCl2, 0.4% NaCl, 0.3% mixture of minerals and vitamins (0.01 g/kg diet of vitamin E) and 0.1% methionine. The vitamin and mineral premix per kilogram contained the following vitamins: A, 4,000,000 IU; D3, 5000,000 IU; E, 16.7 g; K, 0.67 g; B1, 0.67 g; B2, 2 g; B6, 0.67 g; B12, 0.004 g; B5, 16.7 g; pantothinc acid, 6.67 g; biotin, 0.07 g; folic acid, 1.67 g; choline chloride,400 g; minerals: Zn, 23.3 g; Mn, 10 g; Fe, 25 g; Cu,1.67 g; I, 0.25 g; Se, 0.033 g; Mg, 133.4 g (premix produced by Holland Feed Int. Co.). The chemical analysis of the pellets [15] showed that they contained 17.5% crude protein, 14.0% crude fiber, 2.7% crude fat and 2200 kcal digest able energy/kg diet. After two weeks of acclimation, animals were divided into 4 equal groups, 10 rats each. Group 1 served as control, group 2 was administered orally with Fe2O3NPs (5 mg/kg BW; >50 nm), group 3 was treated interpersonal with AgNPs (50 mg/kg BW; >100 nm), and group 4 was administered with the mixture of Fe2O3NPs with AgNPs. The doses of iron oxide nanoparticles and silver nanoparticles were treated every day for 79 days.
Rats were observed carefully during the acclimatization and experimental periods to monitor any animal showing signs of toxicity, stress, physical damage or mortality. At the end of the 79th day of the experimental period, all animals of each group were anaesthetized with diethyl ether and sacrificed. Blood samples were collected from anaesthetized rats in test tubes containing heparin as an anticoagulant and placed immediately on ice. The blood samples were centrifuged at 860 Xg for 20 min for the separation of plasma. The plasma was kept at - 80°C until analyses of the tested parameters. Testes were immediately removed, washed using chilled saline solution (0.9%), and removed the adhering fat and connective tissues. Testes were minced and homogenized (10%, w/v), separately, in ice-cold sucrose buffer (0.25 M) in a Potter–Elvehjem type homogenizer. The homogenates were centrifuged at 10,000 Xg for 20 min at 4 °C, to pellet the cell debris and the supernatant was harvested and stored at −80 °C for the determination of tested parameters. Glutathione peroxidase activity (GPX; EC. 1.1.1.9) in tissues was assayed by the method of Chiu et al. [16]. Reduced glutathione content in tissues was assayed by the method of Jollow et al. [17]. Glutathione-S transferase enzyme activity (GST; EC 2.5.1.18) was determined according to Habig et al. [18]. Superoxide dismutase enzyme activity (SOD; EC 1.15.1.1) in tissues was assayed by the method of Mishra and Fridovich [19]. Catalase enzyme activity (CAT; EC1.11.1.6) in tissues was assayed by the method of Luck [20]. Thiobarbituric acid-reactive substances content in tissues was assayed by the method of Tappel and Zalkin [21]. Total antioxidant capacity in testes was assayed by the method of Koracevic et al. [22]. Nitric oxide (NO) level in tissues was assayed by the method of Montgomery and Dymock [23].
Statistical Analysis
Statistical analysis for all studied parameters were performed using the general linear model (GLM) produced by Statistical Analysis Systems Institute [24]. Results are reported as means ±SE. Duncan's New Multiple Range Test was used to test the significance of the differences between means [25]. Values of p<0.05 were considered statistically significant.
Results and Discussion
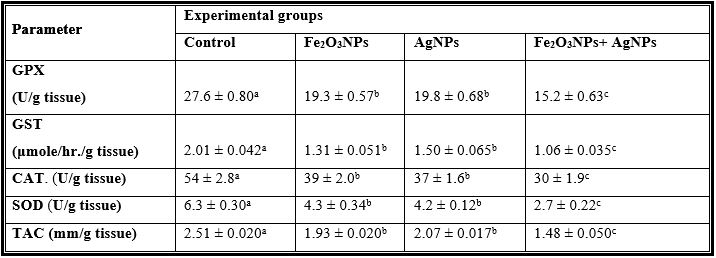
Tables 1 to 4 and Figures 1 to 6 show the activities of the antioxidant enzymes (glutathione peroxidase; GPX, glutathione S-transferase; GST, catalase; CAT, superoxide dismutase; SOD, total antioxidant capacity; TAC), reduced glutathione concentration (GSH), thiobarbituric acid-reactive substances (TBARS) and nitric oxide (NO) in plasma and testes after treatment daily for 79 days of adult male rats with iron oxide nanoparticles (Fe2O3NPs), silver nanoparticles (AgNPs) and their combination. Treatment with Fe2O3NPs, AgNPs and their combination caused significant (P < 0.05) decrease in GPX, GST, CAT, SOD, GSH and TAC and significant (P < 0.05) increase in TBARS and NO compared to control group. The effect of the combination of Fe2O3NPs plus AgNPs was more toxic than each other. SOD and CAT are the two basic subcellular defenses of antioxidant system that counteracts free radicals produced during xenobiotics exposure [26]. SOD catalyses the conversion of superoxide radicals to hydrogen peroxide, while CAT converts hydrogen peroxide into water [27]. Also, SOD catalyzes the dismutation of the superoxide anion (O2.-) into hydrogen peroxide and molecular oxygen, is one of the most important antioxidative enzymes [28]. This suggested that H2O2 generated by SOD was removed by CAT directly. The reduction in the activities of the antiperoxidative enzymes (SOD and CAT) may be due to the increased generation of ROS such assuperoxide and hydrogen peroxide, which in turn leads to the inhibition of the activities of these enzymes [29].
Glutathione peroxidase (GPx) is involved in protecting of cytosol and plasma membrane from lipid peroxidation. In fact, this enzyme transforms lipid hydroperoxides; produced at the membrane level, into less reactive species [30]. The lower enzymatic activity of GPx in treated samples could facilitate increased lipid peroxidation, missing the protective effect of this antioxidant enzyme [31]. The GSH is one of the primary cellular antioxidant defenses against oxidative stress. The cysteine amino acid in glutathione can function as a thiol reducing agent, thus buffering cellular oxidants [32]. The activity of superoxide dismutase (SOD), glutathione peroxidase (GPX) and glutathione reductase (GR) of sperm decreased in toxic materials while thiobarbituric reactive substances (TBARS) levels and nitric oxide were increased [33]. Garrido et al. [34]. reported that human spermatozoa exhibits a capacity to generate ROS and initiates the peroxidation of the unsaturated fatty acids in the sperm plasma membrane, which plays a key role in the etiology of male infertility. ROS are generated mainly in mitochondria during oxidative phosphorylation. Physiologically, cells defend themselves against ROS damage with antioxidant enzymes such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx) and glutathione S-transferase, as well as using non-enzymatic factors such as glutathione to reduce ROS. An imbalance between the level of destructive ROS and the availability of biological systems for detoxification of the reactive species leads to oxidative stress [35].
Nemenqani et al. [36]. Demonstrated that oxidative stress induction is the major toxicological mechanism of ambient NPs. Upon entering the cell, particles may induce intracellular oxidative stress by disturbing the balance between oxidant and antioxidant processes. Excessive oxidative stress may also modify proteins, lipids, and nucleic acids, which further stimulates the antioxidant defense system or even leads to cell death [37]. Nel et al. [38]. Reported that LPO and oxidative stress is one of the more important mechanisms of toxicity related to nanoparticles. Iron oxide nanoparticles cause injury to the cell membrane as indicated by an increased level of lactate dehydrogenase enzyme release. Their results are consistent with the findings of other investigators who demonstrating that metal oxide nanoparticles have the potential to induce DNA damage [39]. The NPs have the potential to interact with the biological system and cause undesirable effects. One of these damaging effects could be the disturbance in the natural balance between oxidative stress and antioxidant defense indices, which in turn can lead to various pathological effects. The oxidative stress has identified as a likely mechanism of nanoparticle toxicity [40]. Production of ROS is a common mechanism causing nanoparticle toxicity. It has been pointed out that the toxicity of NPs is determined by their potency to produce ROS, which is balanced by the antioxidant capacity of an organism to prevent oxidative damage [38]. Alarifi et al. [41]. Reported that iron oxide nanoparticles have cytotoxic and genotoxic effects on MCF-7 cells. The results also revealed that the mode of cell death was apoptosis which was mediated by the ROS-triggered mitochondrial pathway as evidenced by cleavage of caspase-3 activity. Nanoparticles may change the production of ROS and affect antioxidant defenses to induce oxidative stress [42]. Iron oxide nanoparticles induce oxidative stress in MCF-7 cells. The generation of ROS and malondialdehyde (MDA) were found to be increased significantly. On the other hand, superoxide dismutase, GSH, and catalase activities were reduced. The increased levels of ROS have been found to negatively disrupt the balance between oxidation and antioxidant defense systems, which leads to apoptosis via oxidative damage to intracellular proteins and DNA and increased LPO [43]. To counteract the adverse effects of ROS, cells utilize antioxidant enzymes such as superoxide dismutase, GSH, and catalase to remove the redundant ROS. The increased production of ROS and MDA and the reduced activity of the antioxidant enzymes superoxide dismutase, GSH, and catalase suggested that iron oxide nanoparticles caused imbalances between the production and degradation of ROS and induced oxidative stress, changes which may result in cell damage and apoptosis. Free oxygen radical generation and oxidative stress elicit a wide variety of cellular events including DNA damage and apoptosis [44]. Adeyemi and Faniyan [45]. Found that superoxide dismutase levels were inconsistently elevated after exposure to the nanoparticles. This is an inducible enzyme, and elevated levels may indicate the presence of reactive species; a previous report linked elevated superoxide dismutase levels to the presence of oxidative stress [46]. Catalase levels were inconsistently altered after exposure to nanoparticles, as observed previously in broilers [47]. Conversely, silver nanoparticles caused inconsistent reductions in the levels of GST in serum and tissues, perhaps because the nanoparticles have an affinity for thiol groups [48, 49]. The alterations in the levels of these enzymes may represent an adaptive mechanism to offset the stress of exposure. El-Tohamy [50]. Reported that overproduction of ROS can be detrimental to sperm as it is may be associated with male infertility. This could be coincides with the current finding where there was an elevation in the level of free radicals and nitric oxide, and reduction in the antioxidant enzymes and total antioxidant capacity in plasma and the testicular tissue of rats administered with high concentration of IONPs and AgNPs. These results indicate that silver nanoparticles may cause lipid peroxidation and alter antioxidant status in a manner that may cause oxidative stress [45]. The cellular lipid peroxidation indicates that AgNPs induced oxyradicals in the testes tissues. Spermatozoa are susceptible to peroxidative damage because of the high concentration of polyunsaturated fatty acids and a low level of antioxidants [51].
Exposure to silver nanoparticles significantly decreased the levels of GSH in rat plasma and testes. GSH is an antioxidant that can quench free radicals or serve as a substrate for other antioxidant enzymes, such as glutathione peroxidase and glutathione reductase. The decreased levels of GSH after exposure to silver nanoparticles may be due to complexing of silver nanoparticles with thiol groups [48, 52] or to increasing use of GSH to downplay the effect of free radicals after exposure to of the nanoparticles [46]. Foldbjerg et al. [53] reported that apoptosis induced by exposure to AgNPs was mediated by oxidative stress in fibroblast, muscle and colon cells. Ranjbar et al. [54]. Found that antioxidant enzymes activity such as GPx and SOD were used to measure the production of ROS in variouse dose of AgNPs. These data suggest that Ag NP can induce oxidative damage through a ROS-mediated process. However, it remains to be investigated whether Ag NP induce free radicals directly or indirectly through depletion of antioxidant defense mechanisms depending dose e.g. caused by interactions with antioxidant systems [55].
The present study showed that IONPs and AgNPs caused significant increase in the level of nitric oxide (NO) in plasma and testes and this is coincided with the decrease in sperm production and sperm motility; increase the abnormal sperm, changes in steroidogenic enzymes and histopathology of testes. Also, several studies have confirmed the role of NO in modulation of sexual and reproductive function and, it has also been suggested that NO might be involved in different testicular abnormalities, i.e. in inhibiting human sperm motility, in germ cell degeneration and in stress-impaired testicular steroidogenesis [56,57].
It was concluded that results showed significant (P < 0.05) decrease in the antioxidant enzymes (GPX, GST, CAT and SOD) and reduced glutathione (GSH) and total antioxidant capacity (TAC), while significant (P < 0.05) increase in TBARS and NO in plasma and testes of rats treated with Fe2O3NPs, AgNPs and their combination compared to control group.
Table 1: Mean Values ± Se of Plasma Antioxidant Enzymes of Male Rats Treated with Iron Oxide Nanoparticles (Fe2o3nps), Silver Nanoparticles (Agnps) and Their Combination.
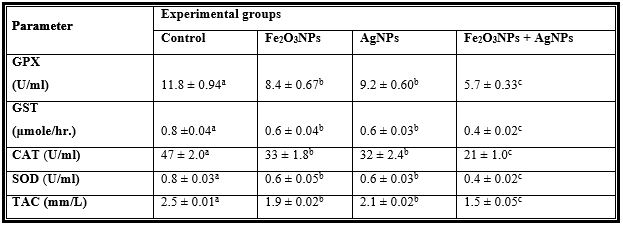
Mean values within a row not sharing a common superscript letters (a, b, c, d) were significantly different,
p < 0.05.
GPX = Glutathione peroxidase, GST = Glutathione S-transferase, CAT = Catalase, SOD = Superoxide dismutase, TAC = Total antioxidant capacity.
Table 2: Mean Values ± Se of Plasma Reduced Glutathione and Free Radicals of Male Rats Treated with Iron Oxide Nanoparticles (Fe2o3nps), Silver Nanoparticles (Agnps) and Their Combination.
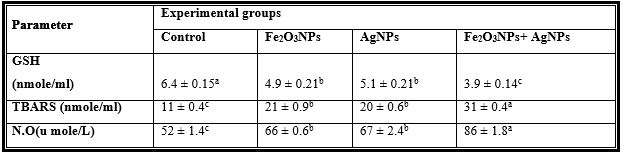
Mean values within a row not sharing a common superscript letters (a, b, c) were significantly different, p < 0.05.
GSH= Reduced glutathione concentration, TBARS= Thiobarbituric acid-reactive substances, NO=Nitric oxide.
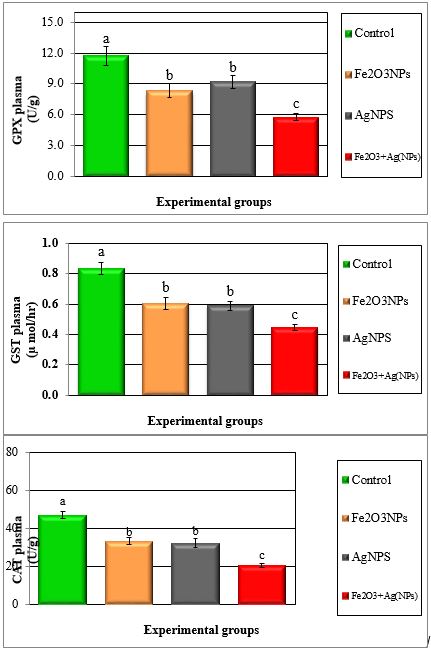
Figure 1: Mean values ± SE in plasma antioxidant enzymes of male rats treated with iron oxide nanoparticles (Fel2O3NPs), silver nanoparticles (AgNPs) and their combination.
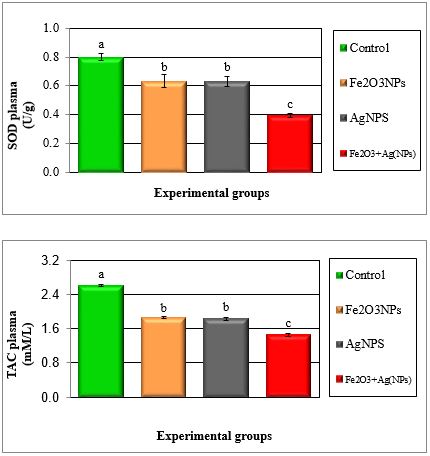
Figure 2: Mean values ± SE of plasma superoxide dismutase and total antioxidant capacity of male rats treated with iron oxide nanoparticles (Fe2O3NPs),silver nanoparticles (AgNPs) andtheir combination.
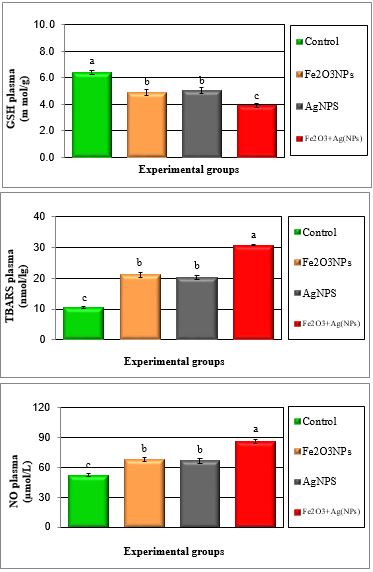
Figure 3: Mean values ± SE of plasma reduced glutathione and free radicals of male rats treated with iron oxide nanoparticles (Fe2O3NPs), silver nanoparticles (AgNPs) and their combination.
Table 3: Mean Values ± Se of Testes Glutathione Peroxidase, Glutathione S-Transferase, Catalase, Superoxide Dismutase and Total Antioxidant Capacityof Male Rats Treated with Iron Oxide Nanoparticles (Fe2o3nps), Silver Nanoparticles (Agnps) and Their Combination.
Mean values within a row not sharing a common superscript letters (a, b, c) were significantly different, p < 0.05.
GPX= Glutathione peroxidase, GST= Glutathione S-transferase, CAT= Catalase, SOD= Superoxide dismutase, TAC= Total antioxidant capacity.
Table 4: Mean Values ± Se of Testes Reduced Glutathione and Free Radicals of Male Rats Treated with Iron Oxide Nanoparticles (Fe2o3nps), Silver Nanoparticles (Agnps) and Their Combination.
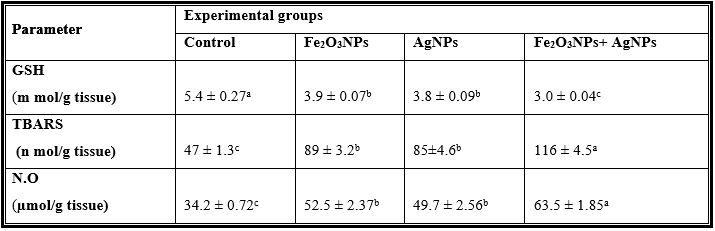
Mean values within a row not sharing a common superscript letters (a, b, c) were significantly different, p < 0.05.
GSH= Reduced glutathione concentration, TBARS= Thiobarbituric acid-reactive substances, N.O=Nitric oxide.
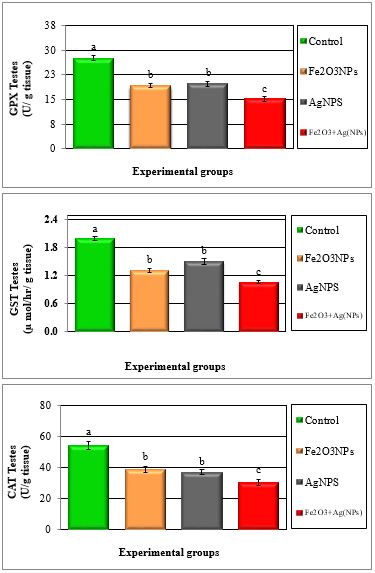
Figure 4: Mean values ± SE of testes glutathione peroxidase, glutathione S-transferase and catalase of male rats treated with iron oxide nanoparticles (Fe2O3NPs), silver nanoparticles (AgNPs) and their combination.
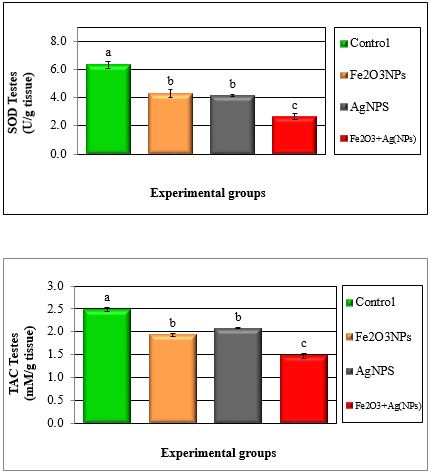
Figure 5: Mean values ± SE of testes of superoxide dismutase and total antioxidant capacity of male rats treated with iron oxide nanoparticles (Fe2O3NPs), silver nanoparticles (AgNPs) and their combination.
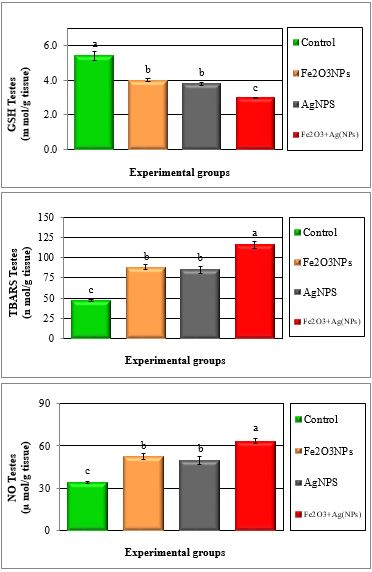
Figure 6: Mean values ± SE of testes reduced glutathione and free radicals of male rats treated with iron oxide nanoparticles (Fe2O3NPs), silver nanoparticles (AgNPs) and their combination.
Acknowledgments
The authors would like to thank the dean of Institute of Graduate Studies and Research and all administrative and teaching staffs at the institute for providing all the facilities required for this study.
Competing Interest
The authors declare that they have no competing interests.
Statement of Ethical Approval
The ethical committee at the Institute of Graduate Studies and Research, Alexandria University, Egypt approved this work.
References
- Wang Z, Li J, Zhao J, Xing B. Toxicity and internalization of CuO nanoparticles to prokaryotic algae Microcystisaeruginosa as affected by dissolved organic matter. Environ. Sci. Technol., 2011; 45: 6032-6040.
- Whitesides GM, The 'right' size in Nanobiotechnology. Nat. Biotechnol, 2003; 21: 1161-1165.
- Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ. Sci. Health C "Environ. Carcinog. Ecotoxicol. Rev, 2009; 27: 1-35.
- Lodhia J, Mandarano G, Ferris N, Eu P, Cowell S, Development and use of iron oxide nanoparticles (Part 1) Synthesis of iron oxide nanoparticles for MRI. Biomed. Imaging Interv, 2009; 6: 1-11.
- Medina C, Santos-Martinez MJ, Radomski A, Corrigan OI, Radomski MW, Nanoparticles pharmacological and toxicological significance. Br J. Pharmacol, 2007; 150: 552-558.
- Kulthong K, Srisung S, Boonpavanitchakul K, Kangwansupamonkon W, Maniratanachote, R., . Determination of silver nanoparticle release from antibacterial fabrics into artificial sweat. Part Fiber Toxicol, 2010; 7: 2-9.
- Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol. Let. 2008; 176: 1-12.
- Ji JH, Jung JH, Kim SS, Yoon JU, Park JD, Choi BS, et al. Twenty-eight-day inhalation toxicity study of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol, 19: 857-871.
- Luoma PV. Cytochrome P450 and gene activation-from pharmacology to cholesterol elimination and regression of atherosclerosis. European. J Clin. Pharmacol, 2008; 64: 841-850.
- Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann MC. In Vitro Cytotoxicity of Nanoparticles in Mammalian Germline Stem Cells. Toxicol. Sci, 2005; 88: 412–419.
- Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. .In Vitro Toxicity of Nano particles in BRL 3A Rat Liver Cells. Toxicol. In Vitro, 2005; 19: 975-983.
- Arora S, Jain J, Rajwade JM, Paknikar KM. Cellular Responses Induced by Silver Nanoparticles: In Vitro Toxicol. Let, 2008; 179: 93-100.
- Szala B, Tátrai E, Nyírő G, Vezér T, Dura G. Potential toxic effects of iron oxide nanoparticles in vivo and in vitro experiments J. Appl. Toxicol, 2011; 32: 446-453.
- Sharma P, Brown SC, Bengtsson N, Zhang Q, Walter GA, Grobmyer SR, et al. Gold-speckled multimodal nanoparticles for noninvasive bioimaging. Chem. Mater, 2008; 20: 6087-6094.
- Official Methods of Analysis of the Association of Official Analytical Agricultural Chemists, 13th ed. Benjamin, Franklin Station, Washington DC, 1990; 587 p.
- Chiu DTY, Stults FH, Tappel AL. Purification and properties of rat lung soluble glutathione peroxidase. Biochim. Biophys.Acta, 1976; 445: 558-566.
- Jollow DJ, Michell JR, Zampaglionic GJR. Bromoibenzene-induced Liver necrosis: Protective role of glutathione and evidence for 3, 4-Bromobenzene oxide as hepatotoxic metabolite. Pharmacology, 1974; 11: 151-169.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione-S transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem., 1974; 249: 7130-7139.
- Mishra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem 1972; 247: 3170-3175.
- Luck H. Catalase. In Method of Enzymatic Analysis MV. Bergmayer ed. Verlag Chemic. Academic Press, New York, NY.1974; 885-895.
- Tappel AL, Zalkin H. Inhibition of lipid peroxidation in mitochondria by vitamine E Arch. Biochem. Biophys.1959; 80: 333-336.
- Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. Clin. Pathol, 2001; 54: 356-361.
- Montgomery HAC, Dymock JF. The determination of nitrate in water. Analyst, 1961; 86: 414-416.
- SAS, Statistical Analysis System. SAS Procedure Guide. Release 6.03 Edition. SAS Institute. Inc., Cary, Nc, U.S.A. 1998.
- Duncan DB. Multiple Range and Multiple (F-test). Biomaterials, 1955; 11: 1-42.
- Messarah M, Boumendjel A, Chouabia A, Klibet F, Abdennour C, Boulakoud MS, et al. Influence of thyroid dysfunction on liver lipid peroxidation and antioxidant status in experimental rats. Exp. Toxicol. Pathol, 2010; 62: 301-310.
- Mansour SA, Mossa AH. Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pestic. Biochem. Physiol., 2010; 96: 14-23.
- Abdelhalim MAK, Al-Ayed MS, Moussa SA. The effects of intraperitoneal administration of gold nanoparticles size and exposure duration on oxidative and antioxidants levels in various rat organs. Pak. J. Pharm. Sci., 2015; 28: 705-712.
- Karthikeyan K, Sarala Bai BS, Devaraj SN. Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats. Int. J. Cardio., 2007; 115: 326-333.
- Hussiena HM, Abdoub HM, Yousef MI. Cypermethrin induced damage in genomic DNA and histopathological changes in brain and haematotoxicity in rats: The protective effect of sesame oil. Brain. Res. Bull., 2013; 92: 76-83.
- Gabbianelli R, Falcioni G, Nasuti C, Cantalamessa F. Cypermethrin-induced plasma membrane perturbation on erythrocytes from rats: reduction of fluidity in the hydrophobic core and in glutathione peroxidase activity. Toxicology, 2002; 175: 91-101.
- Haseeb AK, Adelhalim MAK, Al-Ayed MS, Alhomida AS. Effect of GNPs on glutathione and malondialdehyde levels in liver, lung, heart and kidney of rats in vivo. Saudi J. Biol. Sci., 2012; 19: 461-464.
- Rao MV, Gangadharan B. Antioxidative potential of melatonin against mercury induced intoxication in spermatozoa in vitro. Toxicol. In Vitro, 2008; 22: 935-942.
- Garrido N, Meseguer M, Carlos SC, Pellicer A, Remohi J. Pro-oxidative and anti-oxidative imbalance in human semen and its relation with male fertility. Asian. J .Androl., 2004; 6: 59-65.
- Betteridge DJ. What is oxidative stress? Metabolism, 2000; 49: 3-8.
- Nemenqani D, El-Gharib O, Amany M, Ayman A, Baiuomy R. The Protective Effects of Antioxidant (Vitamin C) Against Hepatic Oxidative Damage Induced by Zinc Oxide Nanoparticals. Intl. Res. J. Appl. Basic. Sci., 2015; 9: 502-509.
- Wang Z, Li J, Zhao J, Xing B. Toxicity and internalization of CuO nanoparticles to prokaryotic algae Microcystisaeruginosa as affected by dissolved organic matter. Environ. Sci. Technol., 2011; 45: 6032-6040.
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science, 2006; 311: 622-627.
- Eom HJ, Choi J. Oxidative stress of silica nanoparticles in human bronchial epithelial cell, Beas-2B. Toxicol. In Vitro, 2009; 23: 1326-1332.
- Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineer enanoparticles. Free Radic. Biol. Med., 2008; 44:1689-1699.
- Alarifi S, Ali D, Alkahtani M, Alhader MS. Iron Oxide Nanoparticles Induce Oxidative Stress, DNA. 2014.
- Damage, and Caspase Activation in the Human Breast Cancer Cell Line. Biol. Trace. Elem. Res., 159: 416–424.
- Srinivas A, Rao PJ, Selvam G, Murthy PB, Reddy PN. Acute inhalation toxicity of cerium oxide nanoparticles in rats. Toxicol. Lett., 2011; 205: 105–115.
- Naziroglu M, Uguz AC, Kocak A, Bal R. Acetaminophen at different doses protects brain microsomal Ca2+-ATPase and the antioxidant redox system in rats. J. Membr Biol., 2009; 231: 57-64.
- Ostrovsky S, Kazimirsky G, Gedanken A, Brodie C. Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res., 2009; 2: 882-890.
- Adeyemi OS, Faniyan TO. Antioxidant status of rats administered silver nanoparticles orally. J. Taib. Univer. Med. Sci., 2014; 9: 182-186.
- Adeyemi OS, Sulaiman FA. Biochemical and morphological changes in Trypanosoma brucei brucei-infected rats treated with homidium chloride and diminazene aceturate. J. Basic. Clin. Physiol. Pharmacol., 2012; 23: 179-183.
- Lovric J, Bazzi HS, Cuie Y, Fortin GR, Winnik FM, Maysinger D. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J. Mol. Med., 2005; 83: 377-385.
- Srivastava M, Singh S, Self WT. Exposure to silver nanoparticles inhibits selenoprotein synthesis and the activity of thioredoxin reductase. Environ. Health , 2012; 120: 56-61.
- Abbas SAR, Abdullah AH, Sada SHA, Ali AK. The effects of gold and silver nanoparticles on transaminase enzymes activities. Int. J. Chem. Res., 2011; 1: 1-11.
- El-Tohamy The mechanisms by which Oxidative Stress and Free Radical Damage produces Male infertility. Life Science Journal 2012; 9(1):674-688 ·
- Vernet P, Aitken R.J, Drevet JR. Antioxidant strategies in the epididymis. Mol. Cell. Endocrinol, 2004; 216: 31 – 39.
- Salma AA, Amer HA, Shaemaa HA, Abdulrahman KA. The effects of gold and silver nanoparticles on transaminase enzymes activities. Int. J. Chem. Res., 2011; 1: 2249-2329.
- Foldbjerg R, Olesen P, Hougaard M, Dang DA, Hoffmann HJ, Autrup H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol lett., 2009; 190: 156-162.
- Ranjbar A, Ataie Z, Khajavi F, Ghasemi H. Effects of silver nanoparticle (Ag NP) on oxidative stress biomarkers in rat. Nanomed. J., 2013; 1: 205-211.
- Piao MJ, Kang KA, Lee IK, Kim HS, Kim S, Choi JY, et al. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. lett, 2011; 201: 92-100.
- Romeo C, Ientile R, Impellizzeri P, Turiaco N, Teletta M, Antonuccio P, et al. Preliminary report on nitric oxide‐mediated oxidative damage in adolescent varicocele. Human Reprod., 2003; 18: 26-29.
- Mehraban D, Ansari M, Keyhan H, Sedighi Gilani M, Naderi G, Esfehani F. Comparison of nitric oxide concentration in seminal fluid between infertile patients with and without varicocele and normal fertile Men. Urology J., 2009; 2: 106-110.

