B-Type Natriuretic Peptide and High Sensitivity C-Reactive Protein Performances in the Screening for Diabetic Nephropathy in Type 2 Diabetic Patients
Ibtissem O1*, Rym B1, Imen S1, Emna T2, Nadia K1, Fatma C1, Meriem Y1 and Melika C1 Huynh Hong Qu2*, Chau Van K1, Nguyen Thanh TN2 and Nguyen DH1
1Department of Endocrinology, Faculty of Medicine of Tunis, La Rabta Hospital, Tunis, University of Tunis El Manar, Tunisia
2Department of Biochemistry, Faculty of Medicine of Tunis, La Rabta University Hospital, Tunis, University of Tunis El Manar, Tunisia
Received Date: 23/09/2020; Published Date: 26/10/2020
*Corresponding author: Ibtissem Oueslati, Department of Endocrinology, Rue Jbel Lakhdar, La Rabta Jebbari 1007, Tunis, Tunisia E-mail: ouesibtissem@gmail.com
Abstract
Introduction: The associations between B-type natriuretic peptide (BNP), high sensitivity C-reactive protein (hs-CRP) and diabetic nephropathy (DN) in patients with type 2 diabetes mellitus remain controversial.
Aim: To analyze the performance of BNP and hs-CRP in the screening for DN in type 2 diabetic patients without renal dysfunction.
Methods: In a cross sectional study, we enrolled type 2 diabetic patients aged less than 65 years and having a glomerular filtration rate > 60 ml/minute/1.73 m2. All patients had plasma BNP and hs-CRP measurements.
Statistical analysis used: A receiver operating characteristic (ROC) curve was used to determine the optimal cut-off values of plasma BNP and hs-CRP in the screening for DN.
Results: Among 69 participants (mean age: 56.7 ±6.9 years, sex ratio (M/F): 1.46), 26 patients (38%) had diabetic nephropathy (DN). BNP level was higher in patients with DN (42.0 ± 42.2 pg/ml vs 19.2 ± 20.7 pg/ml, p=0.04) and was significantly correlated with albuminuria level (r= 0.295, p=0.017). However, hs-CRP level was not significantly different between the two groups. Using BNP and hs-CRP, the area under the ROC curve was 0.702 (p=0.007) and 0.570 (p=0.316), respectively. A BNP level ≥ 15 pg/ml was significantly associated with DN (Odds Ratio= 3.91, p= 0,008). This cut-off value had a sensitivity of 65 % and a specificity of 67 %.
Conclusion: BNP level was positively associated with diabetic nephropathy. However, prospective controlled studies including a large population are needed to confirm these results.
Keywords: Type 2 Diabetes; Diabetic Nephropathy; Biomarker; B-Type Natriuretic Peptide; High Sensitivity C-Reactive Protein
Introduction
Diabetic nephropathy (DN) is a syndrome characterized by diabetic glomerular lesions, a pathological urinary albumin excretion and a loss of glomerular filtration rate in diabetic patients [1]. It represents the first cause of kidney disease in patients starting renal replacement therapy and affects 40% of type 1 and type 2 diabetic patients (T2D) [2]. Furthermore, DN is a risk factor for cardiovascular diseases. So, the diagnosis and the management of DN are important for the prevention of chronic kidney disease (CKD) and its complications.
Brain natriuretic peptide (BNP) is one of the predictive biomarkers of cardiovascular events. In fact, it is secreted as a prohormone from cardiomyocytes in response to volume expansion and ventricular wall stress, and is then released into the circulation following cleavage into active BNP and N-terminal pro-BNP (NT-pro-BNP) fragment. Although the two latter parameters are released in equimolar amounts, NT-pro-BNP is found in higher concentrations in the circulation and has a longer half-life than active BNP, possibly due to differences in its specific degradation [3]. It is also used for the prediction of cardiovascular events in diabetic patients and in patients undergoing dialysis. Moreover, BNP is considered as a biomarker of renal function and can predict the progression of non-diabetic CKD [4]. In DN, some reports have shown that the plasma BNP level is high in DN and may increase depending on the stage of albuminuria [4-6]. However, the role of BNP as a marker of DN has not yet been established. High sensitivity C-reactive protein (hs-CRP), a marker of inflammation, has been reported to be associated with the development of DN and some authors noted an increase in serum Hs-CRP with the degree of albumin excretion rate (AER) and the severity of DN in T2D [7-9]. Nevertheless, there is not conclusive evidence that the development and the progression of DN were associated with high levels of hs-CRP.
The aim of this study was to analyze the performances of BNP and hs-CRP in the screening for DN in Tunisian T2D patients.
Subjects and Methods
Study Protocol and Subjects
A cross-sectional study was conducted among consecutive T2D patients who attended the Endocrinology outpatient department of the Rabta University Hospital in Tunis from March to September 2017.
Type 2 diabetes was diagnosed according to the American Diabetes Association (ADA) criteria [10]. Inclusion criteria were T2D patients aged between 30 and 65 years and having glomerular filtration rate > 60 ml/mn/1.73 m2. Patients with urinary tract infection, history of treated diabetic nephropathy, severe concomitant diseases, inflammatory and or infectious diseases, heart failure or left ventricular hypertrophy, pregnant or nursing women and patients with hypothyroidism or hyperthyroidism were not included. Patients with BNP level ≥ 300 pg/ml and or hs-CRP > 10 mg/l were excluded.
Sixty nine patients were eligible to participate in this study. They were informed about the aim of the study and were included if they had signed a consent form. All procedures performed in our study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Participants were divided into two groups according to the presence of DN. DN was defined by the presence of a pathological excretion of urinary albumin (with repeated measurements) referred to as microalbuminuria if the level is 30–299 mg/24 hours or macroalbuminuria if the level is ≥ 300 mg/24 hours [1].
Clinical and Biochemical Data
Sociodemographic characteristics (age, gender and smoking habits), diabetes mellitus history (diabetes duration, antidiabetic medication, and diabetic complications), comorbidities and drugs use were determined via a structured interview. Cardiovascular disease (coronary heart disease, peripheral artery disease and or stroke) and microangiopathies (retinopathy and or neuropathy) were assessed based on the medical records and/or the admission data. Cardiovascular disease (CVD) was considered present if any of the following criteria was present: history of stroke, history of myocardial infarction, history of coronary angioplasty or coronary bypass surgery or history of peripheral artery disease.
Patients’ weight and height were recorded. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Overweight and obesity were defined as BMI ≥ 25 and ≥ 30 kg/m2, respectively. Blood pressure was measured after a rest period of approximately 15 minutes in a sitting position. High blood pressure was defined as systolic blood pressure ≥ 140 mmHg and or diastolic blood pressure ≥ 90 mmHg or the use of blood pressure lowering medication during the previous two weeks [11].
Blood samples were drawn after an overnight fast of at least 12 hours. Urine was collected over a full 24-hour period. Fasting blood glucose, HbA1c, total cholesterol (TC), triglycerides (TG), HDL-cholesterol (HDLc), serum creatinine, BNP concentration, hs-CRP and albuminuria level were measured. Fasting blood glucose and HbA1c were measured using glucose oxidase method and high performance liquid chromatography (HPLC) method, respectively. TC, TG and HDLc were measured using enzymatic colorimetric methods. The plasma BNP level concentration was measured with specific chemiluminescent enzyme immunoassay using a human BNP kit. Serum hsCRP concentration was determined by a turbidimetric immunoassay method. Urinary albumin rate was assessed by an immuno-nephelometric method. The presence of abnormal albuminuria was confirmed in at least two consecutive samples.
The level of glycemic control was evaluated according to the recommendations of the American Diabetes Association, ADA 2016 [10]. Good glycemic control was defined as HbA1c ≤ 7% for most adult patients or as HbA1c ≤ 8% for patients with a history of severe hypoglycemia, limited life expectancy, advanced microvascular or macrovascular complications, extensive comorbid conditions, or long-standing diabetes in whom the goal was difficult to achieve despite diabetes self-management education, appropriate glucose monitoring, and effective doses of multiple glucose-lowering agents including insulin. Dyslipidemia was defined according to the guidelines of the European Society of cardiology ESC 2016 [12]. Glomerular filtration rate (eGFR) was estimated using the MDRD (Modification of Diet in Renal Disease Study) equation.
Statistical Analysis
All data were analyzed using the Statistical Program for Social Sciences, SPSS version 22.0 (SPSS Inc., Chicago, IL). Continuous variables were expressed as mean ± SD, and categorical variables were expressed as percentages. For independent groups, we used the parametric Student’s T-test for the comparison of means and the Pearson’s Chi-2 test to compare proportions. Receiver operating characteristics (ROC) analysis was used to identify optimal cut-off values of BNP and hs-CRP for the prediction of DN. The area under the ROC curve (AUC) and the 95% confidence interval (95% CI) were used as measures of the discriminative power of the BNP and the hs-CRP. AUC value above 0.80 was considered as excellent. We calculated quartiles of BNP to analyze the association between BNP serum levels and DN. Relative risk analysis was performed using univariate regression analysis. A nominal p value <0.05 was considered statistically significant.
Results
A total of 69 subjects with T2D were enrolled in this study. Their mean age was 56.7 ± 6.9 years [extremes: 36-65] and the sex ratio (men/women) was 1.46. The mean duration of diabetes was 10.6 ± 7.7 years.
DN was diagnosed in 26 patients (38%). The comparison of clinical and biological parameters between patients with DN and those without DN is shown in table 1.
BNP level was positively correlated with albuminuria level (r= 0.45, p<10-3). However, no correlation was found between hs-CRP and albuminuria levels (r=-0.03, p=0.8).
Figure 1 shows a significant difference of BNP level between the different stages of DN (p=0.011).
Figure 2 shows the ROC analysis of BNP level and hs-CRP in the screening for DN. The area under the curve was 0.702 [95% CI: 0.563-0.840], p < 0.007] and 0.57 [95% CI: 0.46-0.69], p =0.316], respectively. According to the ROC analysis, the cut-off level of BNP that best predicted DN was 15 pg/ml, with a sensitivity of 65 % and a specificity of 67 %.
In the crude analysis, compared with BNP levels in the first quartile (BNP ≤9 pg/ml), BNP levels in the fourth quartile (BNP > 32.2pg/ml) were significantly associated with DN (table 2).
Table 1: Clinico-biological characteristic of the study population depending on the presence or the absence of DN.
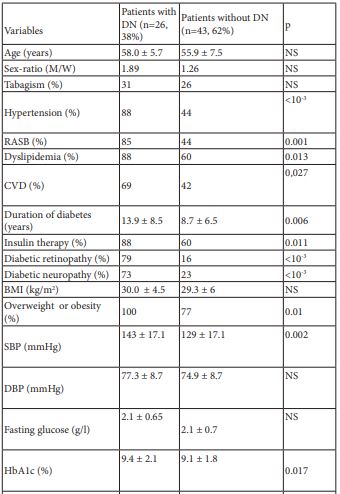
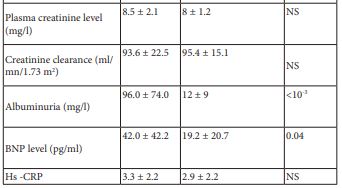
DN: Diabetic Nephropathy
M: Men
W: women
RASB: Rennin-Angiotensin System Blockers
CVD: Cardiovascular Disease
BMI: Body Mass Index
SBP: Systolic Blood Pressure
DBP : Diastolic Blood Pressure
BNP: Type B Natriuretic Peptide
Hs-CRP: High Sensitivity C-Reactive Protein
NS: Not Significant
Table 2: Crude and adjusted odds ratio for nephropathy in relation to plasma level of BNP. (Subjects were divided into quartiles with the following cut-off points.)
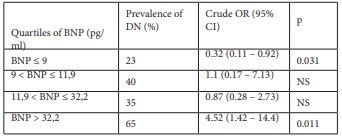
BNP: Type B Natriuretic Peptide
DN: Diabetic Nephropathy
OR: Odds Ratio
CI: Confidence Interval
NS: Not Significant
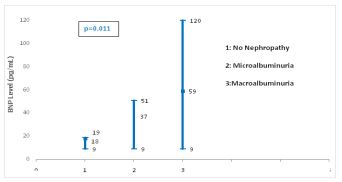
Figure 1: Forest blot values of serum BNP levels according to different nephropathy stages.
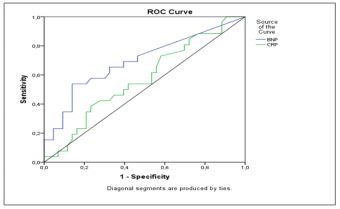
Figure 2: ROC curve analysis of BNP and Hs-CRP levels for the prediction of diabetic nephropathy.
Discussion
In this cross sectional study, BNP level was significantly associated with the presence of DN in patients with T2D with glomerular filtration rate > 60 ml/mn. A BNP threshold value of 15 pg/ml was associated with a sensitivity of 62 % and a specificity of 67% for the prediction of DN. Moreover, a BNP threshold > 32.2 pg/ml was highly associated to DN with an odds ratio of 4.42.
Several studies have evaluated the relationship between BNP and DN. Hamano et al [6] and Seki et al [4] showed that BNP level was significantly higher in the group of patients with DN compared to patients without DN. In a recent study including 687 patients withT2D, Furukawa el al [13] confirmed the existence of a positive association between elevated BNP levels, microalbuminuria and proteinuria. Besides, Seki et al [4, 5], demonstrated that baseline BNP level was an independent predictor of the annual decline in the glomerular filtration rate in T2D.
Furthermore, our study showed a significant difference in BNP levels according to nephropathy stages (p=0.011). Thus, it is possible that BNP may induce by itself the progression of DN. Seki et al [5] explained that elevated endogenous BNP concentrations cause glomerular hyerfiltration which threatens renal function. Indeed, glomerular hyperfiltration, which is often observed in early diabetes, induces glomerular hypertension and stretches mesangial cells mechanically. These stretched cells secrete cytokines that stimulate production of extracellular matrix proteins, accumulation of which promote the progression of renal injury in DN [4]. Many physiological and biochemical factors can be implicated in this association between elevated BNP levels and DN such as cellular hypoxia, increased oxidative stress, activation of renin angiotensin system, acceleration of the sympathetic nervous system, vascular endothelial growth factors (endhotheline) and tumoral necrosis factor α (TNFα) [5, 14]. Many reports demonstrated that the infusion of natriuretic peptide induced proteinuria in diabetes [15] whereas others showed that renal injury can be prevented by the antagonism of natriuretic peptide receptor [16].
In contrast with other reports [17-20], our study has not found a significant association between hs-CRP levels and the presence of DN in T2D patients. In 2002, Stehouwer et al [21] reported for the first time that CRP levels were associated with an increase of urinary microalbumin levels in patients with diabetes. In a cohort of T2D patients, urinary albumin levels increased by 1.02 mg/24 h (95% CI: 1.01–1.27) for each increase of 1 mg/L of CRP over 10 years of follow-up [21]. As well, Pojskić et al [22] indicated that any increase of CRP by 1 mg/L would increase the risk for microalbuminuria by 11.5%. Navarro et al [23] revealed that CRP levels were higher in T2D patients with microalbuminuria or mild proteinuria compared with those with normoalbuminuria. According to Chuengsamarn et al [20], the cut-off point for hs-CRP level for the prediction of DN was 2.10 mg/L.
The exact mechanisms explaining this association are not yet well understood. Some authors suggest that insulin resistance and hyperglycemia may have a role in the development of DN by promoting inflammation and increasing oxidative stress, leading to elevated hs-CRP [9]. On the same way, other authors suggested that pro-inflammatory cytokines and chemokines such as TNF-α, IL-6, intercellular and vascular cellular adhesion molecules (ICAM-1 and VCAM-1), and monocyte chemo-attractant protein-1 (MCP-1) may play a significant role in the development of microvascular diabetic complications such as DN [7, 24]. Thus, high hs-CRP levels may be a part of this complex mechanism. However, further clinical studies should be conducted in order to assess the place of the hs-CRP in the prevention of DN.
Although our study had demonstrated a significant association between BNP level and DN, some limitations should be acknowledged. In fact, this was a single center cross sectional study including a small number of patients. Furthermore, some confounding factors were not considered such as asymptomatic heart disease and beta-blocker use.
Thus, our results couldn’t be generalized to the whole T2D population. Therefore, prospective controlled studies including a large population are needed to confirm these results.
Conclusion
In contrast to hs-CRP, BNP level was associated with the presence and the severity of DN. However, further prospective controlled studies including a large population are needed to confirm the clinical effectiveness of BNP as a biomarker of DN, especially in its early stages.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all participants prior to their inclusion in the study.
Funding Statement
The research did not receive any specific funding.
References:
- Lim AKh. Diabetic nephropathy - complications and treatment. Int J Nephrol Renovasc Dis 2014;7:361-81.
- Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164-76.
- Weber M, Mitrovic V, Hamm C. B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide - Diagnostic role in stable coronary artery disease. Exp Clin Cardiol. 2006;11(2):99-101.
- Seki N, Nishimura M, Matsumoto T, Fukazawa M, Kenmochi T. Relationship between BNP level and renal function in diabetic nephropathy with microalbuminuria. J Diabetes Complications. 2013;27(1):92-7.
- Seki N, Matsumoto T, Fukazawa M. Relationship Between the Brain Natriuretic Peptide (BNP) Level and Prognosis of Diabetic Nephropathy with Microalbuminuria: A 7-Year Follow-Up Study. Horm Metab Res. 2018;50(05):389-96.
- Hamano K, Suzuki J, Nakadaira I, Gonai M. N-terminal fragment of pro brain natriuretic peptide is associated with diabetes microvascular complications in type 2 diabetes. Vasc Health Risk Manag. 2014;10:585-9.
- Liu Q, Jiang C-Y, Chen B-X, Zhao W, Meng D. The association between high-sensitivity C-reactive protein concentration and diabetic nephropathy: a meta-analysis. Eur Rev Med Pharmacol Sci. 2015;19(23):4558-68.
- Shaheer AK. A Comparative Study of High Sensitivity C-Reactive Protein and Metabolic Variables in Type 2 Diabetes Mellitus with and without Nephropathy. J Clin Diagn Res. 2017;11(9):BC01-BC04.
- Hayashino Y, Mashitani T, Tsujii S, Ishii H. Serum High-Sensitivity C-Reactive Protein Levels Are Associated With High Risk of Development, Not Progression, of Diabetic Nephropathy Among Japanese Type 2 Diabetic Patients: A Prospective Cohort Study (Diabetes Distress and Care Registry at Tenri [DDCRT7]). Diabetes Care. 2014;37(11):2947-52.
- American Diabetes Association. Standards of Medical Care in Diabetes-2016 Abridged for Primary Care Providers. Clin Diabetes. 2016;34(1):3-21.
- Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281-357.
- Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37(39):2999-3058.
- Furukawa S, Sakai T, Niiya T, Miyaoka H, Miyake T, Yamamoto S, et al. B-type natriuretic peptide and renal function in Japanese patients with type 2 diabetes mellitus: The Dogo Study. Endocrine Journal. 2017;64(12):1131-6.
- Hanefeld M, Appelt D, Engelmann K, Sandner D, Bornstein S, Ganz X, et al. Serum and Plasma Levels of Vascular Endothelial Growth Factors in Relation to Quality of Glucose Control, Biomarkers of Inflammation, and Diabetic Nephropathy. Hormone and Metabolic Research. 2016;48(08):529-34.
- Zhang PL, Mackenzie HS, Troy JL, Brenner BM. Effects of an atrial natriuretic peptide receptor antagonist on glomerular hyperfiltration in diabetic rats. J Am Soc Nephrol. 1994;4(8):1564-70.
- Moore KB, McKenna K, Osman M, Tormey WP, McDonald D, Thompson CJ. Atrial natriuretic peptide increases urinary albumin excretion in people with normoalbuminuric type-2 diabetes. Irish Journal of Medical Science. 2007;176(2):67-73.
- Liu Q, Jiang C-Y, Chen B-X, Zhao W, Meng D. The association between high-sensitivity C-reactive protein concentration and diabetic nephropathy: a meta-analysis. Eur Rev Med Pharmacol Sci. 2015;19(23):4558-68.
- Shaheer AK. A Comparative Study of High Sensitivity C-Reactive Protein and Metabolic Variables in Type 2 Diabetes Mellitus with and without Nephropathy. J Clin Diagn Res. 2017;11(9):BC01-BC04.
- Liao L, Lei M, Chen H, Wu J, Guo L. [High-sensitive C-reactive protein and Type 2 diabetic nephropathy]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2004;29(6):627-30.
- Chuengsamarn S, Rattanamongkolgul S, Sittithumcharee G, Jirawatnotai S. Association of serum high-sensitivity C-reactive protein with metabolic control and diabetic chronic vascular complications in patients with type 2 diabetes. Diabetes Metab Syndr. 2017;11(2):103-108.
- Stehouwer CDA, Gall M-A, Twisk JWR, Knudsen E, Emeis JJ, Parving H-H. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51(4):1157-65.
- Pojskić L, Hasić S, Tahto E, Arnautović-Torlak V, Pojskić B. Influence of C-reactive protein on the occurrence and assessing of albuminuria severity in diabetics. Med Glas (Zenica). 2018;15(1):10-15.
- Navarro JF, Mora C, Maca M, Garca J. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis. 2003 ;42(1):53-61.
- Elmarakby AA, Abdelsayed R, Yao Liu J, Mozaffari MS. Inflammatory cytokines as predictive markers for early detection and progression of diabetic nephropathy. EPMA Journal. 2010;1(1):117-29.

