Mycarelink Patient Monitors After Pacemaker Implantation in Nigeria
Emmanuel Auchi Edafe1,* and Iseko Iseko Iyoko2
1University of Port Harcourt Teaching Hospital, Port Harcourt, Nigeria
2Cardiocare Multispecialty Hospital, Garki, Abuja, Nigeria
Received Date: 28/01/2024; Published Date: 13/06/2024
*Corresponding author: Emmanuel Auchi Edafe, University of Port Harcourt Teaching Hospital, Port Harcourt, Nigeria
Abstract
Objective: Patients with Medtronic pacemakers were evaluated with Mycarelink monitor. An observational, prospective pilot study with pacemaker patients put on Mycarelink temporarily for a maximum of 2 weeks.
Materials and Methods: This study is descriptive and prospective. It was design to see how Mycarelink can work in the sub-Saharan Africa. It was used temporary to see how Mycarelink can be used to support distant monitoring of patients with pacemakers in the region. Data was collected and analysis with SPSS version 25th.
Results: There were a total of 19 patients with pacemakers enrolled into the study. The new pacemaker’s data were downloaded and transmitted with ease. The Reconditioned pacemaker’s data were not downloaded and transmitted.
Conclusion: The use of Mycarelink is visible in sub-Saharan Africa. This will be of great benefit to patients in the region when this programme start. Its use for reconditioned devices needs to be evaluated and worked out with the pacemaker producing companies.
Keywords: Mycarelink; Patient monitor; Pacemaker; Sub-Saharan Africa
Introduction
Remote patient morning is part of the standard of care for patients with cardiac devices in the developed countries of Europe and north America [1-4]. This standard of care has not been part of the routine care in Nigerian patients who had the device implanted in the country. However, some patients who travelled oversea to the countries of Western Europe and North American come home with these patients’ homecare monitors. Some get malfunction while other still work after coming home to Nigeria. There has not been any local site where these patient homecare monitors being used in at a local site of implantation in Nigeria despite the increase number of device implant yearly, hence the need for this pilot study. In this pilot study, we evaluated the temporary use of Mycarelink patient monitor locally to see the visibility of these care and the challenges in starting the programme.
Methods
This was a pilot study of 19 patients who received dual chamber pacemakers that we temporarily put on my care link patient monitor. The present study was a very short-term follow-up of 2 weeks, exploring the ability of the new and Reuse pacemaker in Mycarelink data base and remove monitoring of the patients. After ethical approval, the study span through 1st May to 31st August 2023.
Patients were included in the study if the following criteria were meant: (i) Indication for pacemaker implantation based on the European Society of Cardiology 2021 pacing guideline (ii) they were at least 18 years old, (iii) they understand and correctly carried out the home auto-monitoring or a caregiver performed this action and and to return it in two weeks (iv) They provided the signed informed consent to participate in the study.
Patients were excluded if (i) they had been Patients below 18 years, (ii) they had any other cardiac device implanted or (iii) they did not agree to participate.
The steps in registration of the patients into Mycarelink included the Figure 1. Enter the pacemaker type [e.g., Sphera, Azure, Adaptive, etc.] and serial number in the hospital care link programme data base. Enter the Mycarelink patient monitor in the data base and link it to the patient pacemaker. This is to ensure compatibility before data transmission. Connect the monitor to source of electricity and the device will automatically switch on. Then follow the instruction by doing what the Mycarelink monitor tells you to do.
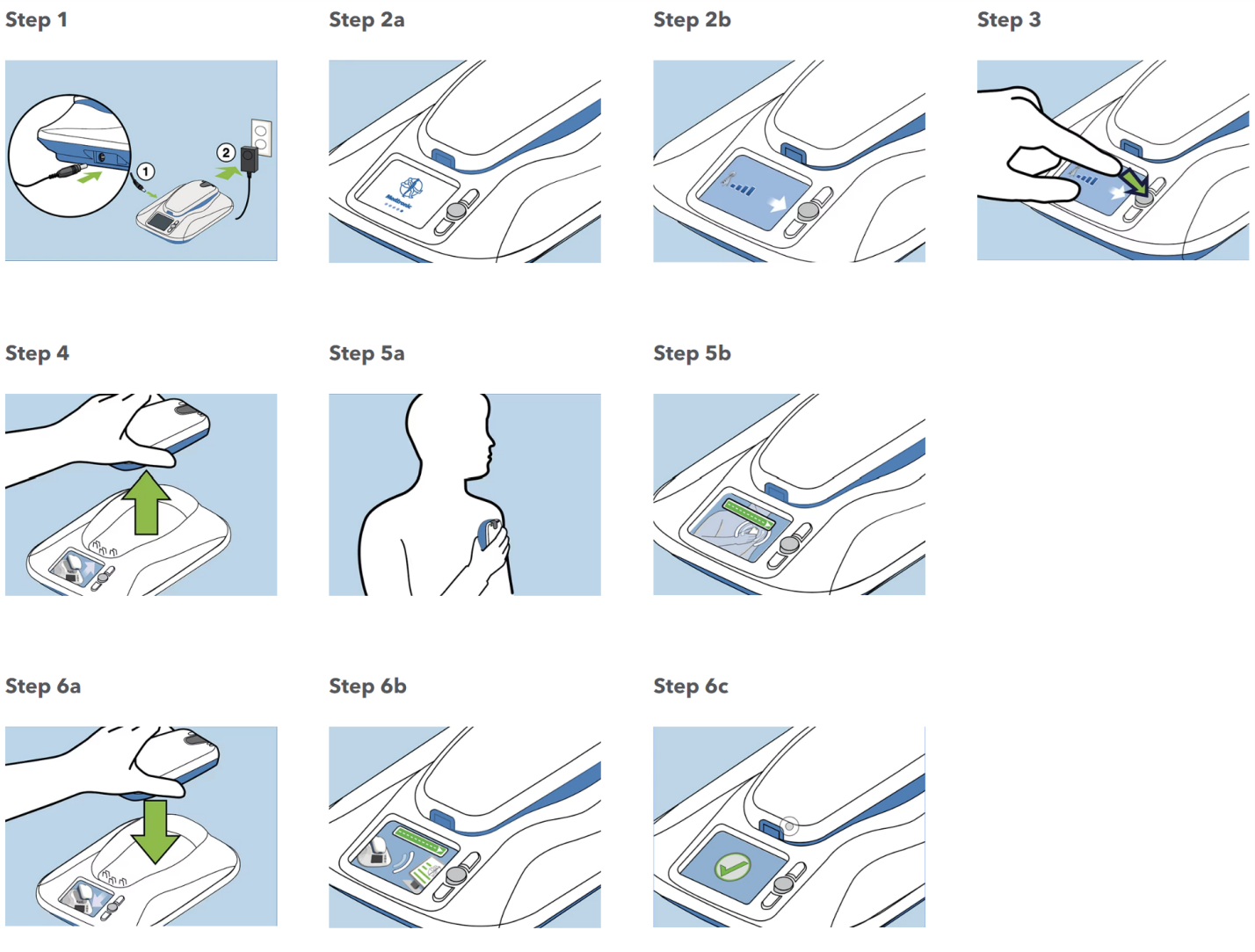
Figure 1: Steps in the setup of MyCareLink monitor.
The Mycarelink monitor comes ready to use as in Figure 1. Use it with the following steps1. Take it out of the box and place it close to a power outlet near where you sleep. Using the power cord that came with your new monitor, plug the circular end into the jack on the side of the monitor and the other end into the wall outlet. 2. Once powered, an animated display screen on the monitor provides easy to follow step by step instructions. The monitor will let you know if it is not receiving a cellular signal. If that's the case, move the monitor to an area where the signal is stronger, close to a window, for example, but still within 10 feet of where you sleep. To start the first transmission, press the accept button next to the animated display screen. Now, remove the reader from the top of the monitor and place the reader over your heart device. Then green bar lets you know that the reader is positioned correctly and reading your device. If you see this on the screen, it's telling you to reposition the reader for better information gathering. When the reading is complete, you'll be prompted to put the reader back on the monitor base. This allows the monitor to send the heart device information so it can be viewed by the doctor at the clinic. There will be another green bar indicating the device information is being sent, followed by a check mark and audible confirmation when the transmission is complete. The home screen will now display the date of your last successful transmission.
This temporary remote monitoring was used for two weeks, and the data were analysed using SPSS version 25.
Results
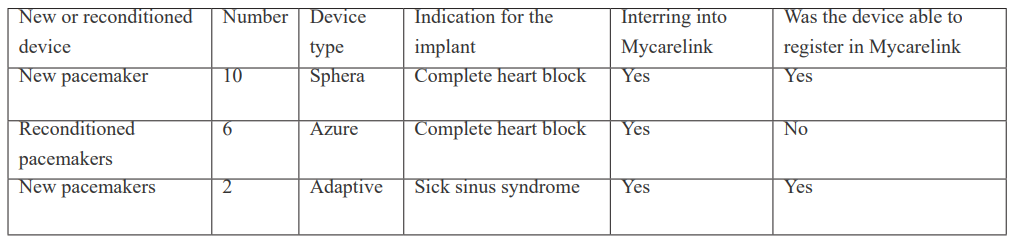
There were 19 patients with dual chamber pacemakers. The total number of men were 12 [63.2%]. The age ranged from 40 to 96 years with average aged of 72.5±6years. Table 1 showed the patient device characteristics. All the new devices data were download and registered into Mycarelink smoothly but the Reuse [Reconditioned] pacemakers. The new pacemaker’s data were downloaded with transmitted with ease. The Reconditioned pacemaker’s data already exist in the data base, reassigning the device to medically indigent [ those that can afford a new device] means that the information cannot be easily transmitted.
Table 1: Patient device characteristic.
Discussion
In the sub-Saharan Africa, patients with an implanted pacemaker (PM) undergo periodic check-ups to verify the accurate functioning of the device and to get it reprogrammed for optimal therapy if needed [5-9]. This is down when the patient presents physically to the pacemaker clinic. The role of pacemaker Telemedicine via Mycarelink is not routine in the West and East Africa.
The continuous adjustment of pacing parameters can afford important benefits to the patient and improve the effectiveness of treatment [10-15]. Regular follow-ups play a crucial role in the quick detection of technical and clinical faults that may appear during the functioning of the pacemaker [7,8]. This significantly increases the workload of the staff of institutions that manage these devices [16,17]. In this pilot study, we showed that remote pacemaker monitoring is possible and it is good option for the Nigeria and the sub-Saharan Africa.
In 2021 the European Society of Cardiology guidelines for pacing recommended remote patient monitoring for those with implanted pacemaker [18]. Remote monitoring (RM) or telemonitoring of patients with PMs is rapidly increasing over the world but this is not so in Nigeria and other countries of the sub-Saharan Africa [19,20]. RM is the vanguard of the personal ‘big data’ revolution in telemedicine or telehealth services [19].
Remote monitoring provides direct care at patients’ homes and to the people with chronic conditions, where it is used to improve patients’ feeling of safety and empower them to manage their condition and prevent hospitalization. In our study, all the new pacemaker’s data were download and transmitted with the Medtronic Mycarelink but patients with reconditioned devices were not.
With Mycarelink patient monitor, the patient will be able to enjoy remote care and monitor in the sub-Saharan Africa. This will remove periodic visit to the pacemaker clinic. In this pilot study, we noticed that the reconditioned pacemakers were data were not downloaded and transmitted. So, this need to be worked out with the device companies to ensure medically indigent patients in the region enjoy the service.
Some hindrances to Mycarelink patient monitors in the region include source of light and power generation to constantly power the device and patients in village areas where there is not communication setting to reach out to them.
Conclusion
The use of Mycarelink is visible in sub-Saharan Africa. This will be of great benefit to patients in the region when this programme start. Its use for reconditioned devices need to be evaluated and worked out with the pacemaker producing companies.
References
- Mond HG, Proclemer A. The 11th World Survey of Cardiac Pacing and Implantable Cardioverter – Defibrillators: Calendar Year 2009–A World Society of Arrhythmia’s Project. Pacing and Clinical Electrophysiology, 2011; 34(8): 1013-1027.
- Glickman SW, Schulman KA, Peterson ED, Hocker MB, Cairns CB. Evidence-based perspectives on pay for performance and quality of patient care and outcomes in emergency medicine. Annals of Emergency Medicine, 2008; 51(5): 622-631.
- William J, DesRochers L, Enguidanos E, Fite D, Fitz J, Freess D, et al. Patient Satisfaction, An Information Paper. American College of Emergency Physicians: Emergency Medicine Practice Committee, 2011.
- Heidbüchel H, Lioen P, Foulon S, Huybrechts W, Ector J, Willems R, et al. Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator. Europace, 2008; 10(3): 351-357.
- Costa PD, Reis AH, Rodrigues PP. Clinical and economic impact of remote monitoring on the follow-up of patients with implantable electronic cardiovascular devices: An observational study. Telemedicine and e-Health, 2013; 19(2): 71–80.
- Kallinen LM, Hauser RG, Tang C, Melby DP, Almquist AK, Katsiyiannis WT, et al. Lead integrity alert algorithm decreases inappropriate shocks in patients who have Sprint Fidelis pace-sense conductor fractures. Heart Rhythm, 2010; 7(8): 1048–1055.
- Volosin K, Stadler RW, Wyszynski R, Kirchhof P. Tachycardia detection performance of implantable loop recorders: results from a large ‘real-life’ patient cohort and patients with induced ventricular arrhythmias. Europace, 2013; 15(8): 1215–1222.
- Santini M, Ricci RP, Lunati M, Landolina M, Perego GB, Marzegalli M, et al. Remote monitoring of patients with biventricular defibrillators through the CareLink system improves clinical management of arrhythmias and heart failure episodes. Journal of Interventional Cardiac Electrophysiology, 2009; 24(1): 53–61.
- Bikou O, Licka M, Kathoefer S, Katus HA, Bauer A. Cost savings and safety of ICD remote control by telephone: a prospective, observational study. Journal of Telemedicine and Telecare, 2010; 16(7): 403–408.
- Varma N, Epstein AE, Irimpen A, Schweikert R, Love C. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up the Lumos-T Safely Reduces Routine Office Device Follow-Up (TRUST) trial. Circulation, 2010; 122(4): 325–332.
- Crossley G, Boyle A, Vitense H, Sherfesee L, Mead RH. Trial design of the clinical evaluation of remote notification to reduce time to clinical decision: The clinical evaluation of remote notification to reduce time to clinical decision (CONNECT) study. American Heart Journal, 2008; 156(5): 840–846.
- Akar JG, Bao H, Jones P, Wang Y, Chaudhry SI, Varosy P, et al. Use of remote monitoring of newly implanted cardioverter-defibrillators: insights from the patient related determinants of ICD remote monitoring (predict RM) study. Circulation, 2013; 128(22): 2372–2383.
- Klersy C, De Silvestri A, Gabutti G, Regoli F, Auricchio A. A meta-analysis of remote monitoring of heart failure patients. Journal of the American College of Cardiology, 2009; 54(18): 1683–1694.
- Udo EO, van Hemel NM, Zuithoff NP, Barrett MJ, Ruiter JH, Doevendans PA, et al. Incidence and predictors of pacemaker reprogramming: Potential consequences for remote follow-up. Europace, 2013; 15: 978-983.
- Andreozzi E, Gargiulo GD, Fratini A, Esposito D, Bifulco P. A Contactless Sensor for Pacemaker Pulse Detection: Design Hints and Performance Assessment. Sensors, 2018; 18: 2715-2723.
- Deering TF, Clair WK, Delaughter MC, Fisher WG, Garlitski AC, Wilkoff BL, et al. A Heart Rhythm Society Electrophysiology Workforce study: Current survey analysis of physician workforce trends. Heart Rhythm, 2010; 7: 1346-1355.
- García-Fernández FJ, Osca Asensi J, Romero R, Fernández Lozano I, Larrazabal JM, Martínez Ferrer J, et al. Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: A long-term randomized trial (RM-ALONE) Eur. Heart J, 2019; 40: 1837-1846.
- Glikson M, Nielsen JC, Kronborg MB, Auricchio MYA, Barbash IM, Barrabe JA, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. European Heart Journal, 2021; 42: 3427-3520.
- Villamil CA, Landinez SF, Lopez DM, et al. A mobile ECG system for the evaluation of cardiovascular risk. In: Hoerbst A, Hackl WO, DeKeizer N, Prokosch HU, HercigonjaSzekeres M and DeLusignan S (eds) Exploring complexity in health: an interdisciplinary systems approach. 228. Popayan, Colombia: Univ Cauca, Telemat Engn Res Grp, 2016; pp.210–214.
- Caso F, Del Puente A, Girolimetto N, et al. Improving telemedicine and in-person management of rheumatic and autoimmune diseases, during and after COVID-19 pandemic outbreak. Definite need for more rheumatologists. Response to: ‘Can telerheumatology improve rheumatic and musculoskeletal disease ser’. Ann Rheum Dis, 2020.

