A Beginner's Guide to Droplet Digital PCR (ddPCR): Revolutionizing DNA and RNA Quantification in Molecular Diagnostics
Riya Kadam1, Pradnya Joshi1, Mahika Shetty2, Niral Panchal1, Rachna Rumde1, Prachi Bapat1, Mamta Gurav1 and Omshree Shetty1,*
1Molecular Pathology division, Department of Pathology, Tata Memorial Hospital, Homi Bhabha National Institute, India
2South Fayette High School (Pittsburgh, Pennsylvania), USA
Received Date: 20/01/2025; Published Date: 20/02/2025
*Corresponding author: Omshree Shetty, Molecular Pathology division, Department of Pathology, Tata Memorial Hospital, Homi Bhabha National Institute, India
Abstract
Droplet Digital PCR (ddPCR) has emerged as a transformative technology in molecular diagnostics, offering unparalleled sensitivity and precision in nucleic acid quantification. By partitioning samples into thousands of droplets, ddPCR enables a digital approach to DNA and RNA analysis, overcoming limitations of traditional PCR methods. This mini-review highlights key advancements and applications of ddPCR in oncology, including its utility in detecting circulating tumor DNA (ctDNA), copy number variations (CNVs), and epigenetic biomarkers. The technology's ability to identify rare genetic events and monitor tumor heterogeneity has significantly impacted cancer diagnosis, treatment, and monitoring. Moreover, ddPCR's role in non-invasive liquid biopsies and its application in emerging fields such as CAR-T therapy monitoring and tumor microbiome analysis demonstrate its broad clinical potential. Despite challenges such as standardization and cost, ongoing advancements in multiplexing and automation promise to expand ddPCR's scope, further enhancing its contribution to personalized medicine and molecular oncology.
Keywords: Digital PCR; Minimal residual Disease; Clinical diagnostics; Precision Oncology; Liquid Biopsy
Introduction
Droplet Digital Polymerase Chain Reaction (ddPCR) represents a significant advancement in the field of molecular biology, allowing for highly sensitive and precise quantification of nucleic acids. The history of ddPCR is intertwined with the evolution of polymerase chain reaction (PCR), which was invented by Kary Mullis in 1983 and revolutionized molecular biology by enabling the amplification of specific DNA sequences [1]. However, as PCR technology advanced, limitations in sensitivity and quantification became apparent, leading researchers to seek more precise methods for nucleic acid analysis. The concept of ddPCR emerged as a solution to these limitations. Unlike traditional PCR, which amplifies DNA in a single reaction tube, ddPCR partitions the sample into thousands of tiny droplets, each containing individual or few DNA molecules. This droplet-based partitioning technique creates a large number of isolated PCR reactions, allowing for a digital approach to DNA quantification.
Early work in the field of digital PCR laid the groundwork for ddPCR. Digital PCR was initially developed in the 1990s as a concept wherein DNA samples were diluted to the point that each reaction contained a single molecule of DNA, thus enabling precise quantification [2]. However, this early form of digital PCR was not practical for large-scale or routine applications due to technical and logistical constraints. The development of ddPCR in the late 2000s and early 2010s addressed these limitations by using microfluidic technology to create droplets, dramatically increasing the number of partitions and enabling high-throughput digital PCR.
This technology had a significant impact on several fields, including cancer research, virology, genetics, and infectious disease diagnostics, where precise quantification of DNA or RNA is critical. One of the key benefits of ddPCR is its ability to detect rare genetic events and quantify low-abundance targets with high accuracy. This capability has led to widespread adoption in clinical research and diagnostics. For example, ddPCR is now used in cancer research to identify and quantify circulating tumor DNA (ctDNA) in blood samples, providing a minimally invasive method for monitoring tumor progression and treatment response [3]. It is also employed in virology to measure viral loads with high precision, aiding in the management of infectious diseases like HIV and COVID-19. As ddPCR technology continues to evolve, new applications and refinements are emerging, solidifying its role as a powerful tool in molecular biology.
Principles and Technology of ddPCR
One of the key advantages of ddPCR over conventional PCR lies in its partitioning strategy. In conventional PCR, amplification occurs within a bulk sample, producing an overall signal that reflects the collective presence of target molecules. This approach often overlooks the need to consider statistical significance in the analysis. In contrast, ddPCR partitions the sample into thousands of discrete droplets before amplification. Importantly, the random distribution of molecules across droplets, governed by Poisson statistics plays a critical role in the analysis ensuring more accurate and reliable results even for low-abundance targets.
Poisson statistics play a key role in quantifying the concentration of the target molecules. Since the microfluidics technology partitions the sample into multiple droplets the target molecule is randomly distributed among these droplets. The probability that a certain partition will contain certain copies of the target is governed by the possession statistics [4].
After the PCR amplification of the target molecule, the droplets are classified as either positive (with at least one target molecule) or negative (no target molecules). The proportion of negative droplets provides insight into how the molecules were distributed. The ratio of positive to total droplets is calculated using Poisson statistics, and the concentration of the target molecule in the original sample can be estimated. The following formula is commonly used:
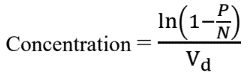
Where, P is the number of positive droplets, N is the total number of droplets, and Vd is the volume of the droplets.
The concentration of the target molecule is reported in terms of copies per microlitre or other relevant units, providing a sensitive and accurate measure of the target’s abundance (in the form of fractional abundance) in the original sample.
Principles and Technology of ddPCR
ddPCR has become increasingly important in cancer research, particularly in the study of solid tumors. This highly sensitive technology is instrumental in the detection and quantification of ctDNA, offering researchers and clinicians a powerful tool for non-invasive cancer monitoring by analyzing ctDNA in a patient's blood [4]. ddPCR provides insights into the genetic makeup of tumors and helps in detecting early signs of cancer recurrence or progression. This capability has significant implications for early diagnosis and monitoring of minimal residual disease (MRD) after treatment [5]. ddPCR detects the progression of the disease and MRD in individuals with chronic myeloid leukemia (CML). When it comes to identifying low amounts of BCR/ABL(P210) transcripts, a marker of CML, ddPCR is a more sensitive technique than conventional RT-qPCR. According to the study, RT-qPCR only identified MRD in five of the 61 CML patients, whereas ddPCR was able to identify MRD in 11 of them. Additionally, in four out of ten CML patients that were monitored, ddPCR was able to identify disease progression before RT-qPCR. According to these findings, ddPCR provides a more precise and sensitive way to track MRD and the course of the disease in CML patients [6]. Study done by Guerrini et. Al. found that ddPCR outperformed traditional RT-qPCR in detecting the B-RAF V600E mutation, identifying it in more patients and earlier in disease progression. These results highlight the potential of ddPCR as a valuable tool for monitoring minimal residual disease and disease progression in Hairy Cell Leukemia patients [7]. Solid tumors are often genetically diverse, with a range of mutations and variations. ddPCR assay can be utilized to identify these mutations with remarkable precision, aiding in the understanding of tumor heterogeneity and allowing for more personalized treatment strategies. For instance, mutations in genes like KRAS, EGFR, or BRAF can be detected, guiding the selection of targeted therapies [8].
A study by Alberto Verlicchi and group demonstrated that ctDNA analysis via ddPCR at key treatment stages, including pre- and post-chemotherapy, provides prognostic insights and improves the detection of recurrence, particularly when monitored longitudinally every three months post-therapy.This aspect of ddPCR is particularly relevant in the context of personalized medicine, where treatment decisions are based on specific genetic profiles [9].
Circulating Tumor cells (CTCs) is a minimally invasive alternative to traditional tissue biopsies allowing for real-time monitoring of tumor dynamics. Low abundance of CTCs in blood bloodstream leads to a low yield of DNA from the CTCs and hence requires techniques with higher sensitivity to detect the biomarkers. One of the studies has documented the detection of KRAS mutations in CTCs from blood samples of colorectal cancer (CRC) patients predicting those found in the tumor has become feasible due to the sensitivity of ddPCR [10].
Several studies have also demonstrated the utility of the ddPCR in detecting the Copy Number Variation (CNV) in the various types of cancers guiding clinical decisions. For example, Her2/neu Amplification detection in breast and gastric cancers in FFPE (Formalin fixed paraffin embedded tissue) extracted DNA. Higher accuracy in the detection of HER2/neu gene amplification in tumors having heterogeneity as well as low IHC scores and intermediate fluorescent signals reported by the Fluorescent In-situ Hybridization (FISH) technique can be effectively achieved through ddPCR technology. ddPCR has been applied to detect CNVs in melanoma, with a focus on genes like RREB1, CDKN2A, MYC, and MYB. The technology demonstrated high concordance with Chromosomal Microarray Analysis (CMA), a gold-standard technique for CNV detection [11].
Epigenetic biomarkers play a crucial role in the tumor pathology such as in tumor classification, understanding the resistance mechanisms and understanding the prognosis of the diseases. Multiple studies have demonstrated the utility of the ddPCR technology in detecting the Methylation biomarkers. A group of researchers evaluated the performance of novel epigenetic methylation biomarkers for detecting CRC using ddPCR, demonstrating high sensitivity and specificity in both tissue and plasma samples. It identified three key markers (C9orf50, KCNQ5, and CLIP4) that could detect early-stage CRC with over 70% sensitivity in plasma samples and 100% specificity in healthy controls [12].
A study by Paola Campomenosi and colleagues demonstrated the effectiveness of droplet digital PCR (ddPCR) in accurately detecting and quantifying miRNAs in lung cancer patients. Extracellular vesicles (EVs), particularly exosomes, are increasingly recognized as important biomarkers across various cancers. These nanoscale particles carry diverse biomolecules, including microRNAs (miRNAs), proteins, and lipids, which mirror the biological state of their cells of origin. The high sensitivity and precision of ddPCR make it an essential tool for analyzing EV-associated miRNAs, offering significant potential to enhance cancer diagnosis, prognosis, and treatment monitoring [13].
Studies have demonstrated that ddPCR can accurately monitor chimeric antigen receptor T- cell (CAR-T) kinetics, playing a crucial role in the monitoring and management of CAR-T cell therapies. ddPCR allows for the detection of CAR transgenes, enabling the quantification of CAR-T cell proliferation dynamics and helping clinicians assess the therapeutic response or early relapse [14].
ddPCR has also been used to study the tumor microbiome by quantifying the bacterial DNA in the tissue. It paves the way to identify potential biomarkers and gaining a deeper understanding of the role of bacteria associated with tumors [15].
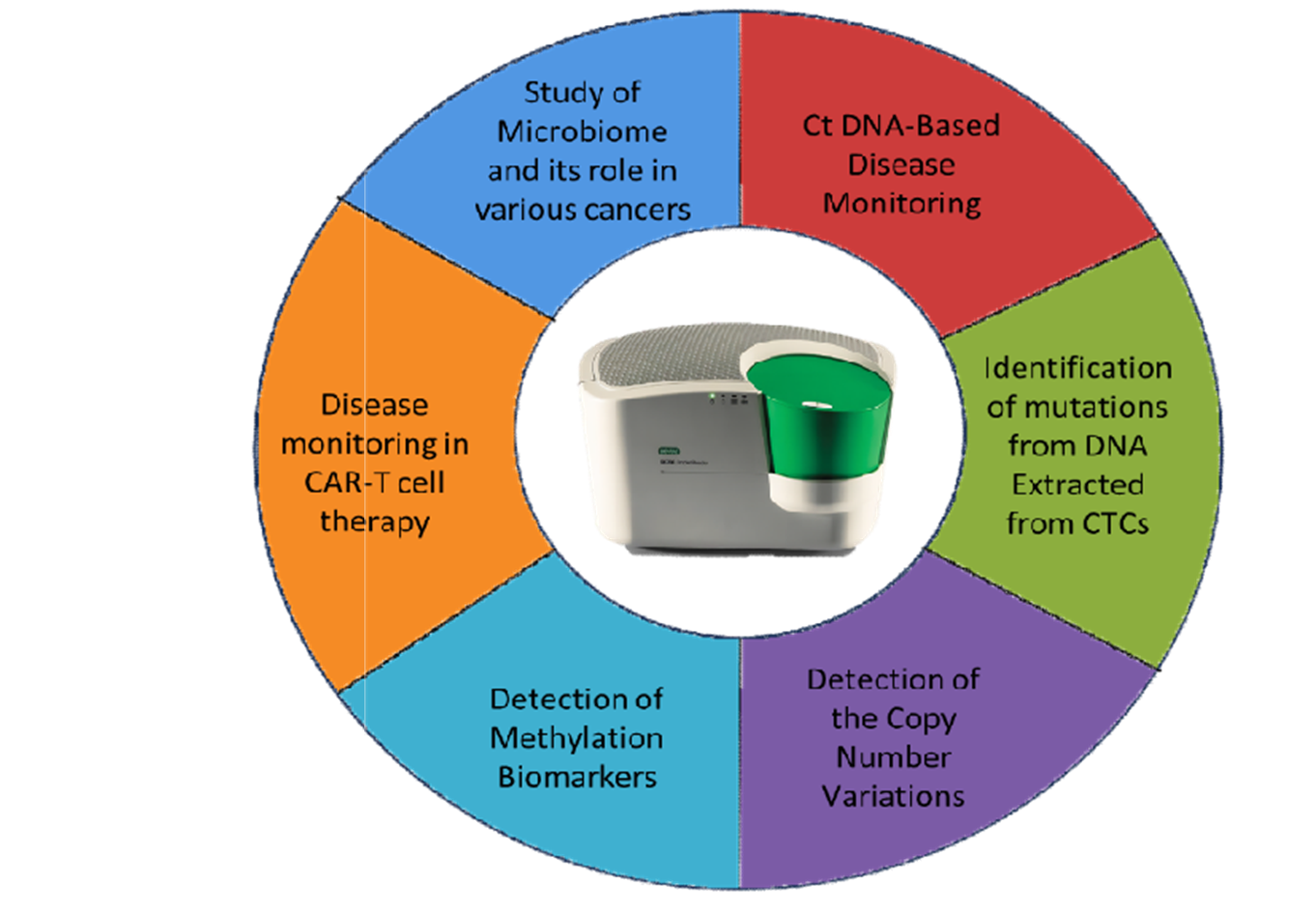
Figure 2: Applications of ddPCR in the field of Oncology.
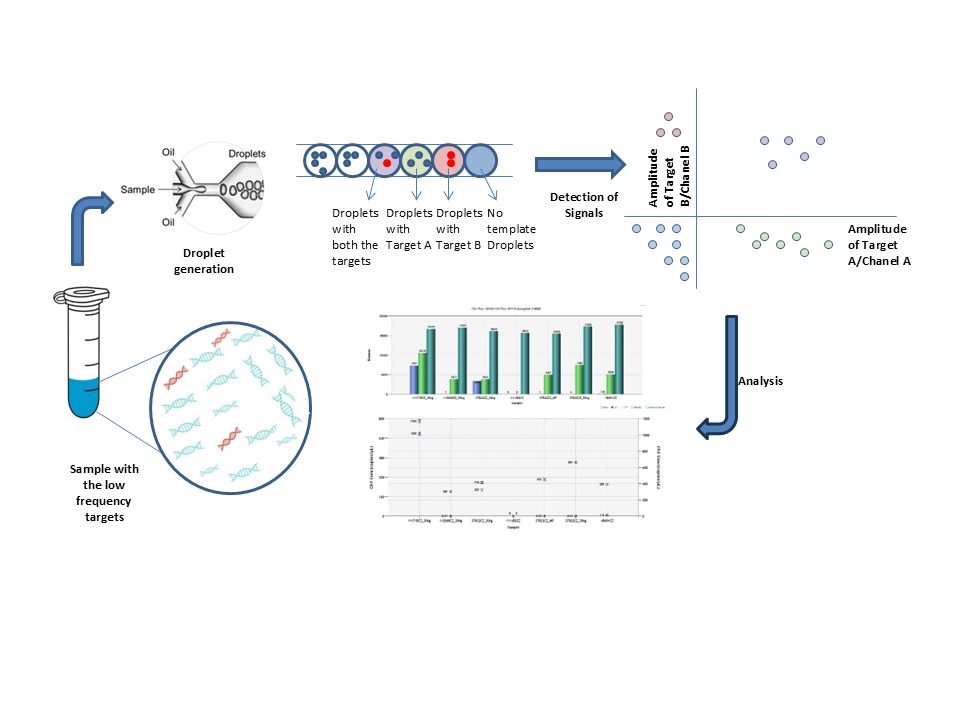
Figure 1: Principle of ddPCR.
Advantages and Limitations of ddPCR
The major advantage of the technology over other conventional is the precise quantification of the target molecules which higher sensitivity and specificity making it ideal for the clinical utility. Technology also provides an exact quantification of the target molecules without requirement of the reference standards leading to the higher accuracy. The partitioning of the sample into thousands of droplets reduces the effects of PCR inhibitors reducing the rate of failure in complex samples.
Despite its advantages, ddPCR also faces challenges, such as standardization across different laboratories and the cost associated with the technology. Ensuring consistent results is essential for its broader adoption in clinical settings. Additionally, further research is needed to explore the full spectrum of ddPCR applications in solid tumors, including its potential to identify new biomarkers for cancer detection and treatment.
Future Prospects of ddPCR in Oncology
ddPCR represents a transformative advancement in the detection and quantification of biomarkers in solid tumors. Its high sensitivity and precision enable robust applications in molecular diagnostics and personalized medicine, improving patient stratification and treatment monitoring. Future innovations, including multiplexing capabilities and automation, are poised to enhance the utility of ddPCR, driving significant progress in the field of molecular diagnostics and the evolution of personalized healthcare.
Author Contributions
Conceptualization: Omshree Shetty
Funding Acquisition: NA
Investigation: Omshree Shetty, Mamta Gurav, Prachi Gogte
Project Administration: Omshree Shetty
Resources: Molecular Pathology Laboratory, Tata Memorial Hospital,Parel,Mumbai
Supervision: Omshree Shetty
Writing-original draft preparation: Mahika Shetty, Pradnya Joshi, Riya Kadam, Omshree Shetty
Writing- Review & editing: Omshree Shetty, Mamta Gurav, Prachi Gogte, Niral Panchal, Rachna Rumde
Competing Interests: All the authors have declared no competing interest.
Grant Information: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sector.
Acknowledgement: We sincerely thank Division of Molecular Pathology, Department of Pathology, Tata Memorial Hospital, Mumbai for their support and resources that facilitated the preparation of this review. We are especially thankful to the broader scientific community for their revolutionary work in advancing digital PCR technology and its implications in oncology.
References
- Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51 Pt 1:263-273. doi:10.1101/sqb.1986.051.01.032
- Morley Digital PCR: A brief history. Biomol Detect Quantif. 2014;1(1):1-2. Published 2014 Aug 15. doi:10.1016/j.bdq.2014.06.001
- Boonstra PA, Gietema JA, Suurmeijer AJH, et al. Tyrosine kinase inhibitor sensitive PDGFR? Mutations in GIST. Two cases and review of the literature. Oncotarget. 2017;8(65):109836-109847. doi:10.18632/oncotarget.22663.
- Zhang S, Zhu L, Xia B, et al. Epidermal growth factor receptor (EGFR) T790M mutation identified in plasma indicates failure sites and predicts clinical prognosis in non-small cell lung cancer progression during first-generation tyrosine kinase inhibitor therapy: a prospective observat. Cancer Commun. 2018;38(1). doi:10.1186/S40880- 018-0303-2
- Sallman DA, DeZern AE, Steensma DP, et al. Phase 1b/2 Combination Study of APR- 246 and Azacitidine (AZA) in Patients with TP53 mutant Myelodysplastic Syndromes (MDS) and Acute Myeloid Leukemia (AML). Blood. 2018;132(Supplement 1):3091- 3091. doi:10.1182/blood-2018-99-119990
- Wang WJ, Zheng CF, Liu Z, et al. Droplet digital PCR for BCR/ABL(P210) detection of chronic myeloid leukemia: A high sensitive method of the minimal residual disease and disease Eur J Haematol. 2018;101(3):291-296. doi:10.1111/ejh.13084
- Guerrini F, Paolicchi M, Ghio F, et al. The droplet digital PCR: A new valid molecular approach for the assessment of B-RAF V600E mutation in hairy cell leukemia. Front Pharmacol. 2016;7:363. doi:10.3389/fphar.2016.00363
- Xu Z, Li Y, Wang L, et al. Efficacy of third-generation epidermal growth factor receptor-tyrosine kinase inhibitors in advanced NSCLC with different T790M statuses tested via digital droplet polymerase chain reaction ddPCR and next-generation sequencing. Expert Rev Anticancer Ther. 2024;24(3-4):183-192. doi:10.1080/14737140.2024.2334807
- Verlicchi A, Canale M, Chiadini E, et al. The Clinical Significance of Circulating Tumor DNA for Minimal Residual Disease Identification in Early-Stage Non-Small Cell Lung Life. 2023;13(9):1915. doi:10.3390/life13091915.
- Liu J, Lian J, Chen Y, et al. Circulating Tumor Cells (CTCs): A Unique Model of Cancer Metastases and Non-invasive Biomarkers of Therapeutic Front Genet. 2021;12 :734595. doi:10.3389/fgene.2021.734595.
- McFadden JR, Syku M, Barney RE, et al. A Novel Method to Detect Copy Number Variation in Melanoma: Droplet Digital PCR for Quantitation of the CDKN2A Gene, a Proof-of-Concept Study. Am J Dermatopathol. 2023;45(7):454-462. doi:10.1097/DAD.0000000000002436.
- Jensen SØ, Øgaard N, Ørntoft MW, et al. Novel DNA methylation biomarkers show high sensitivity and specificity for blood-based detection of colorectal cancer-a clinical biomarker discovery and validation study. Clin Epigenetics. 2019;11(1):158. Published 2019 Nov 14. doi:10.1186/s13148-019-0757-3.
- Campomenosi P, Gini E, Noonan DM, et al. A comparison between quantitative PCR and droplet digital PCR technologies for circulating microRNA quantification in human lung cancer. BMC Biotechnol. 2016;16(1):1-10. doi:10.1186/s12896-016-0292-
- Wiedemann G, Bacher U, Joncourt R, et al. A Comprehensive ddPCR Strategy for Sensitive and Reliable Monitoring of CAR-T Cell Kinetics in Clinical Int J Mol Sci. 2024;25(16):1-17. doi:10.3390/ijms25168556.
- Leoni C, Vinci L, Marzano M, et al. Endometrial Cancer: A Pilot Study of the Tissue Microorganisms, 2024; 12(6): 1090. doi:10.3390/microorganisms12061090.

