Omeprazole-Domperidone Combination Therapy: An Open-label, Prospective, Multicenter, Observational, Patient Reported Outcome Study in Patients with Gastroesophageal Reflux Disease (PROGRESS-2)
Banerjee Rithwik1,*, Itha Srivenu2, Jain Suresh3, Tandan Manu4, Kulkarni Sandeep5, Petare Anup6, Veligandla Krishna Chaitanya6, Rathod Rahul6, Dhanaki Gauri6, Mane Amey6 and Kotak Bhavesh6
1Department Medical Advisor, Dr. Reddy’s Laboratories Ltd. Hyderabad, India
2Cygnus Institute of Gastroenterology, Sardar Patel Nagar, India
3Digestive endoscopy clinic, Opposite YMCA, India
4Asian Institute of Gastroenterology, India
5Sahyadri Hospital, Erandvane, India
6Department of Medical Affairs; Dr Reddy's Laboratories Ltd, India
Received Date: 24/07/2023; Published Date: 14/12/2023
*Corresponding author: Banerjee Rithwik, MBBS, MD; Department Medical Advisor, Dr. Reddy’s Laboratories Ltd. Hyderabad, Telangana, India
Abstract
Objectives: To assess the improvement of symptoms, quality of life, and overall treatment satisfaction with Omeprazole-Domperidone combination therapy in patients with Gastroesophageal Reflux Disease (GERD).
Methods: In this prospective, open-label, multi-centric, observational study, patients were prescribed Omeprazole-Domperidone combination therapy for a period of 4 weeks.
Results: 181 patients were enrolled in the study. The improvement in symptoms, quality of life, and treatment satisfaction was observed on day 14 and day 28 as assessed by PAGI-SYM scores, PAGI-QoL scores, and TSQM respectively. During the study period, there were no safety concerns.
Conclusions: This combination of Omeprazole and Domperidone has proved to be beneficial, in not only reducing the GERD symptoms but also improving the quality of life and treatment satisfaction of patients and the treatment was well tolerated.
Keywords: Omeprazole; Domperidone; Gastroesophageal reflux disease; Combination therapy; Symptom improvement; Quality of life; Treatment satisfaction
Introduction
Background
Gastroesophageal Reflux Disease (GERD) is usually characterized as a burning sensation in the chest that can extend to the neck, throat, and face; it is worsened by bending or lying down. Heartburn may be the presenting symptom of a heterogeneous group of abdominal disorders related to GERD, non-erosive reflux disease (NERD), and Functional Dyspepsia (FD). These symptoms have a detrimental impact on patient's lives, limiting their ability to carry out activities of daily living as well as giving rise to disruption of physical, social, and emotional well-being [1-5]. A recent study of GERD disclosed that patients with predominant heartburn symptoms had more severely impaired daily activity, including sleep interruption, eating or drinking problems, and work interferences, than those with regurgitation-predominant symptoms [5]. A recent systematic review and meta-analysis revealed that the global pooled prevalence of GERD was 13.98% and it varied greatly according to region [6]. Within the GERD population, the frequency of heartburn varied widely, from those who experience heartburn as infrequently as once per year to those who experience heartburn almost daily [7]. During treatment for GERD symptoms, an effective way to assess changes in symptom severity and frequency is to gauge whether these changes have a beneficial impact on a patient's well-being by using patient-reported outcome measures (PRO). PROs rely on patients' perceptions of outcomes, rather than clinician or investigator assessments [8]. PROs have a uniquely important contribution to make towards both understanding the effect of heartburn on patients' lives and evaluation of treatment efficacy [9]. Primary care physicians continue to experience problems managing heartburn, and there is evidence that patients and clinicians perceive the severity and impact of symptoms differently making PROs an essential component of evaluating clinical treatments [10].
Omeprazole is commonly used to treat GERD symptoms. Omeprazole is a proton pump inhibitor that suppresses gastric acid secretion by specific inhibition of the H+ /K+ -ATPase enzyme system at the secretory surface of the gastric parietal cell. By acting specifically on the proton pump, omeprazole blocks the final step in acid production, thus reducing gastric acidity. There is limited data in scientific literature assessing the impact of omeprazole + domperidone on patient-reported outcomes such as frequency and severity of heartburn symptoms, work productivity, and treatment satisfaction in real-world settings in India. Therefore, the main objective of this clinical study was to assess the impact of Omeprazole and Domperidone combination therapy on the improvement of symptoms, quality of life, and overall treatment satisfaction in patients suffering from GERD at week two and week four post-baseline in a multicenter observational study in India.
Methods
This Post-Marketing Surveillance (PMS) study was designed as an open-label, prospective, multi-centric, observational study in patients with GERD. Patients who met the study eligibility criteria and agreed to participate in the study by signing the informed consent form were prescribed. Omeprazole plus Domperidone combination and followed for four weeks. Patients of either sex, with a diagnosis of GERD, who were ≥18 years and ≤65 years were eligible for the study. They were included if they had been prescribed Omeprazole and Domperidone combination therapy as routine clinical management as per the investigator's discretion. Patients who were hyper-sensitive to any Proton Pump Inhibitor (PPI) including omeprazole in the past and had participated in an investigational drug or investigational device study within 30 before the start of the study were excluded. Additional exclusion criteria included the use of histamine receptor blockers over-the-counter antacids, anticholinergics, cholinergic, spasmolytics, opiates, sucralfate, prokinetics, antibiotics, or bismuth compounds within 14 days prior to the start of the study; any medical condition according to the principal investigator which could have interfered with the treatment and made the patient ineligible for participation in the study; not having sufficient educational level and proficiency in reading and writing in their local language to be capable of reliably completing study questionnaires, as judged by the physician; women who were pregnant or breastfeeding. Patients were allowed to withdraw from the study at any time at their request, or they could be withdrawn at any time at the discretion of the investigator or sponsor for safety, behavioral, or administrative reasons. If a patient did not return for a scheduled visit, every effort was made to contact the patient and document the outcome. Reasons for withdrawal were appropriately documented.
The primary endpoint was to evaluate the symptom improvement score using the Patient Assessment of Gastrointestinal Disorder Symptom Severity Index (PAGI-SYM) [11]. Questionnaire on day 14. The secondary endpoints were to evaluate- the symptom improvement score using the PAGI-SYM questionnaire on Day 28; [12]. The impact on the quality of life of patients using the Patient Assessment of Upper Gastrointestinal Disorder-Quality of Life (PAGI-QOL) questionnaire on Day 28; patient’s overall treatment satisfaction on Day 14 using the Treatment Satisfaction Questionnaire of Medication (TSQM); patient’s overall treatment satisfaction on Day 28 using the Treatment Satisfaction Questionnaire of Medication (TSQM) [13].
Statistical Analysis
A sample size of 174 achieved a 90.14% power to detect a difference (P1-P0) of -0.1083 using a one-sided binomial test. The target significance level was 0.05. Parametric data were presented as means with Standard Deviation (SD), whereas nominal and discrete data were presented as numbers with percentages. Data for the PAGI-SYM, PAGI-QOL, and TSQM scales were computed as total scores for respective domains and presented as means with SD and Standard Error of Means (SEM). Minimum, maximum, and interquartile ranges were also presented. Changes from baseline in the scores on days 14 and 28 were computed as paired differences and presented as means with SD along with 95% confidence intervals for the change. Descriptive statistics were presented for different parameters. Pairwise comparisons of scores (baseline versus day 14 and baseline versus day 28) were analyzed for differences using the Wilcoxon test (nonparametric), whereas the Friedman test was used for the comparison of baseline scores with day 14 and day 28 scores for the total scores of PAGI-SYM and PAGI-QOL. Scores for TSQM (patient satisfaction) were presented as descriptive statistics for the different domains and total scores.
Analyses were done using two-sided tests with alpha 0.05 (95% confidence levels). All the data were entered into a Microsoft Office Excel (Office version 365) spreadsheet and checked for errors and discrepancies. Data analysis was done using Windows-based ‘Stata Version 13, StataCorp LLC, US).
Results
A total of 181 patients (140 males and 41 females) were enrolled in the study. The patient demographics and baseline characteristics are indicated in Table 1. The mean age of participants was 41.17 years and the most common comorbid condition presented at baseline was diabetes (89%), and regurgitation was observed in all patients followed by heartburn (75.7%). Improvements in symptoms (nausea, vomiting, postprandial fullness, bloating, upper abdominal pain, lower abdominal pain, and heartburn) were observed on days 14 (Table 2) and 28 (Table 3). PAGI-SYM scores reduced significantly at day 14 (32.31 to 20.17; p<0.0001) and at day 28 (32.31 to 8.12; p<0.0001) as compared to the baseline scores (Figure 1). While assessing quality of life, domain scores for daily activities (p<0.0001), clothing (p<0.0001), diet (p<0.0001), relationships (p<0.0001) and psychological (p<0.0001) showed significant improvements from baseline at day 14 and day 28. The PAGI-QOL total scores decreased at day 14 (43.96 to 23.14; p<0.0001) and day 28 (43.96 to 12.69; p<0.0001) as compared to the baseline (Figure 2).
Table 1: Baseline characteristics of patients (n=181).
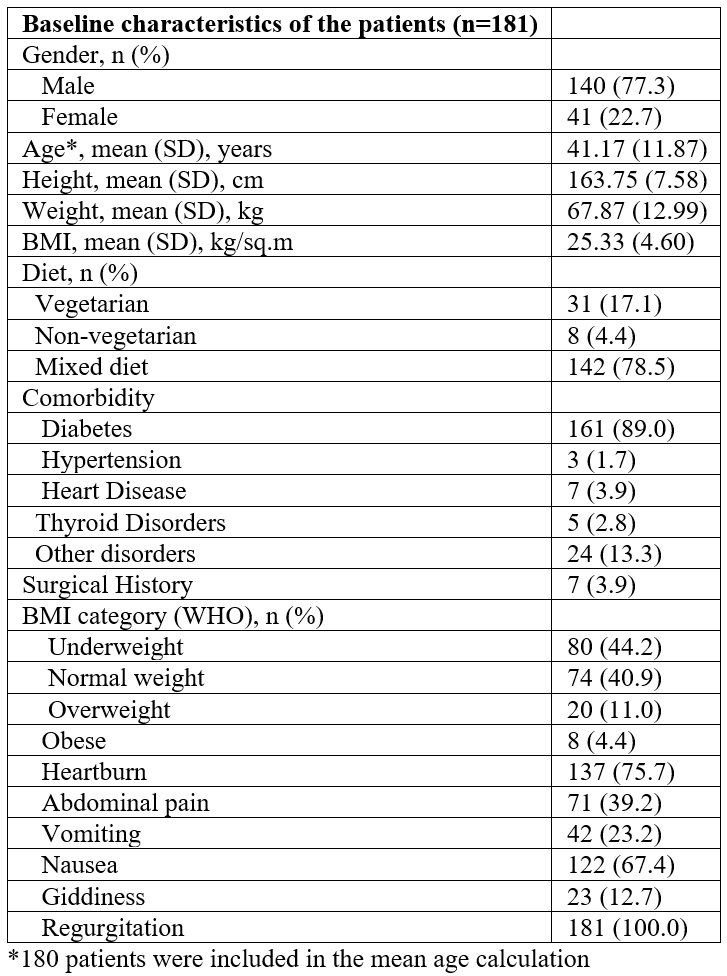
Table 2: Change in PAGI-SYM scores at day 14.

Table 3: Change in PAGI-QOL scores at baseline, and day 28.
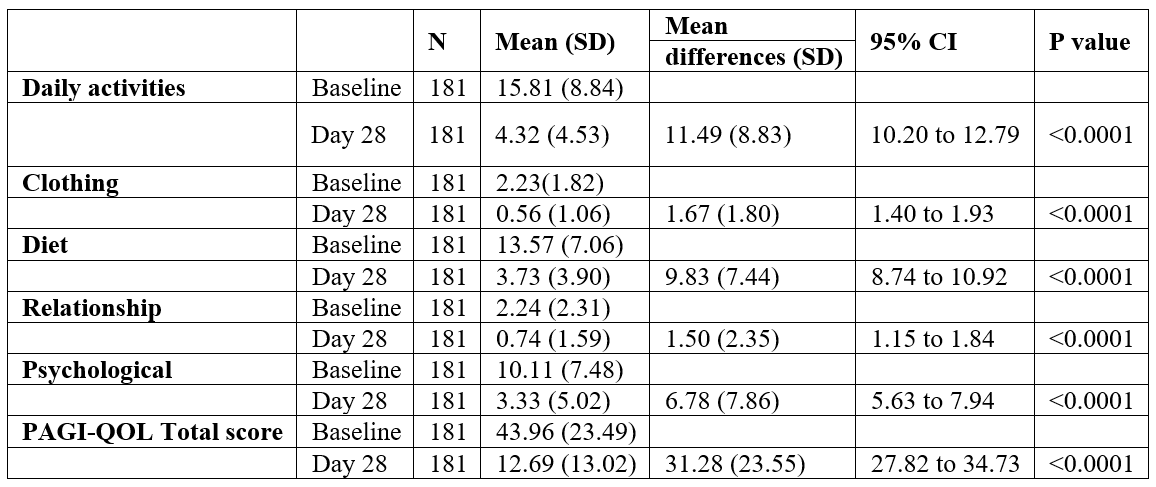
TSQM was a brief questionnaire for patients to rate their satisfaction with the current treatment. The scores for TSQM were higher on day 28 than on day 14 for all domains of effectiveness, convenience, and global satisfaction (Table 4). The total TSQM scores were higher on day 28 (50.12) than on day 14 (40.93) which suggests that the current therapy improves treatment satisfaction over a period. The adverse events of interest were gastrointestinal intolerance, abdominal pain, diarrhea, nausea vomiting, heartburn, giddiness, and regurgitations. However, no adverse events were reported by any of the patients over the 4-week therapy.
Table 4: Patient’s overall treatment satisfaction (TQSM).
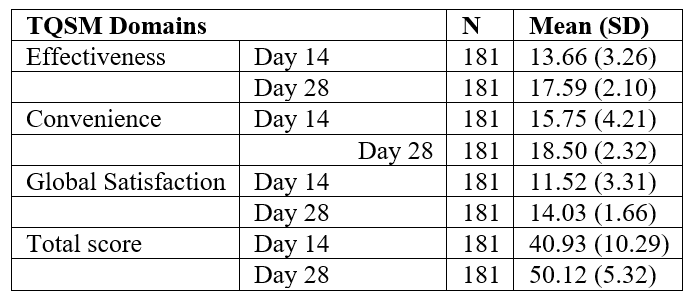
Figure 1: PAGI-SYM total scores at baseline, day 14, and day 28 (n=181).
(PAGI-SYM: Patient Assessment of Gastrointestinal Disorder Symptom Severity Index)

Figure 2: PAGI-QOL total scores at baseline, day 14, and day 28 (n=181).
(PAGI-QOL: Patient Assessment of Upper Gastrointestinal Disorder-Quality of Life)
Discussion
GERD is caused as a result of reflux of stomach contents, giving rise to distressing sensations and/or problems like heartburn or acid indigestion [14]. PPIs are a widely prescribed class of agents for GERD management. It has been reported in a randomized controlled trial by Richter JE, that omeprazole is significantly better in complete resolution of heartburn as a GERD symptom, compared to placebo [20mg omeprazole group (48%), 10mg omeprazole group (27%) and placebo (5%)]. (10) In addition, omeprazole 20 mg showed early, adequate, and long-lasting reflux symptom improvement in Japanese patients with reflux esophagitis who had the CYP2C19 PM phenotype [15]. A prokinetic agent such as Domperidone enhances esophageal peristalsis and improves gastric emptying which further augments the action of PPIs and efficiently relieves GERD symptoms [16,17]. Findings from our study suggest that Omeprazole and Domperidone, when prescribed as a combination therapy, lead to improvement in symptoms such as nausea, vomiting, postprandial fullness, bloating, upper and lower abdominal pain, and heartburn. These findings are supported by findings from a study by Ndraha S et al. which also reported that Omeprazole and Domperidone combination therapy was beneficial in GERD patients. A difference in reduction (7.5 ± 5.9 vs 4.6 ± 3.3) of FSSG scores (frequency scale for the symptoms of GERD) was observed in the combination therapy group [16]. Furthermore, findings from an open-label study conducted in Belarus also showed that combination therapy led to considerably more heartburn-free days [18]. A meta-analysis of 12 Randomized Controlled Trials (RCTs) evaluated the efficacy and safety of PPIs (omeprazole, esomeprazole, and pantoprazole) in combination with Domperidone, and concluded that a significant decrease in global GERD symptoms, therefore deeming this combination to be safer for patients with GERD [19].
Findings from our study also exhibited improved quality of life in patients and greater treatment satisfaction at the end of 4 weeks. Existing evidence indicating improved quality of life and treatment satisfaction with this combination is scarce; however, a meta-analysis by Ren et al. highlights the partial improvement in patients’ quality when prokinetics are added to the PPI therapy. Furthermore, a linear drop in PAGI-SYM scores from baseline to 14 as well as 28 days was observed indicating a significant improvement in the severity and frequency of GERD symptoms such as nausea, vomiting, postprandial fullness, bloating, heartburn, and abdominal pain. A similar trend was observed in PAGI-QOL scores on days 14 and 28, which indicates that the patient's quality of life improved considerably following treatment. Lastly, the fact that the overall TSQM values increased from day 14 to day 28 shows that combination enhances treatment satisfaction over time.
Although this study showed promising results over 4 weeks, certain limitations need to be kept in mind while reviewing the results. First, the study was conducted without a comparator group for the Omeprazole and Domperidone combination. The inclusion of a comparator group may prove the effectiveness of this combination. Second, the small sample size may prevent the generalizability of our results. Thus, in future studies, a larger sample size is recommended. The enrolment of participants for our study reveals a gender disparity wherein the distribution of males to females was approximately 80:20. Consequently, it remains to be seen if a fairly equal balance between the genders would impact our results. Lastly, our study timeline was 4 weeks which is short to capture the follow-up visits as compliance with the treatment may dwindle over a period. In the future, a study with a larger sample size and longer follow-ups is required to understand the improvement of symptoms and quality of life over extended periods. In conclusion, we found significant improvements in symptom scores, quality of life, and treatment satisfaction for these patients. Thus, this combination of a PPI and a prokinetic agent has proved to be beneficial, in not only reducing the GERD symptoms but also improving the quality of life and treatment satisfaction of patients.
Key Takeaways:
- Significant improvements in GERD symptoms (PAGI-SYM) were observed on day 14 and day 28 following the start of combination treatment
- Patients demonstrated significant improvements in quality of life on day 14 and day 28 as indicated by PAGI-QOL scores
- Patients demonstrated greater satisfaction with Omeprazole and Domperidone therapy as indicated by TSQM scores on day 28
Author Contributions (if more than one author):
- Acquisition of data, or analysis and interpretation of data, critical review of manuscript: Itha, Srivenu; Jain, Suresh; Tandan, Manu; Kulkarni, Sandeep
- Conception and design of the study, analysis, and interpretation of data, critical review of manuscript: Petare, Anup; Banerjee, Ritwik; Veligandla Krishna Chaitanya; Rathod, Rahul; Dhanaki, Gauri; Mane, Amey; Kotak, Bhavesh
Competing Interests: None
Grant Information: The study was funded by Dr. Reddy’s Laboratories Ltd.
Acknowledgments (optional): The authors are thankful to PharmEDGE India for the manuscript writing support and Clinsearch Healthcare Solutions Pvt. Ltd. for the statistical analysis and medical writing support.
Conclusion
In this case, due to the features discussed above, we concluded that bidirectional tachycardia due to digital intoxication was caused by two ectopic foci, one supraventricular and the other ventricular.
Conflict of interest: None to declare.
Commemorate: The author respectfully commemorates all our colleagues who lost their lives in the Kahramanmaraş/Turkey earthquake as one of Kahramanmaraş's natives.
References
- Junghard O, Wiklund IK. “Effect of baseline symptom severity on patient-reported outcomes in gastroesophageal reflux disease,” Eur J Gastroenterol Hepatol, 2007; 19(7): pp. 555–560. doi: 10.1097/MEG.0b013e328133f2d1.
- Wiklund I. “Review of the Quality of Life and Burden of Illness in Gastroesophageal Reflux Disease,” Digestive Diseases, 2004; 22(2): pp. 108–114. doi: 10.1159/000080308.
- Wahlqvist P, Karlsson M, Johnson D, Carlsson J, Bolge SC, Wallander M-A. “Relationship between symptom load of gastro-oesophageal reflux disease and health-related quality of life, work productivity, resource utilization and concomitant diseases: survey of a US cohort,” Aliment Pharmacol Ther, 2008; 27(10): pp. 960–970. doi: 10.1111/j.1365-2036.2008.03671.x.
- Lee S-W. “Comparison of presentation and impact on quality of life of gastroesophageal reflux disease between young and old adults in a Chinese population,” World J Gastroenterol, 2011; 17(41): p. 4614. doi: 10.3748/wjg.v17.i41.4614.
- Lee S-W. “Impact of body mass index and gender on quality of life in patients with gastroesophageal reflux disease,” World J Gastroenterol, 2012; 18(36): p. 5090. doi: 10.3748/wjg.v18.i36.5090.
- Nirwan JS, Hasan SS, Babar Z-U-D, Conway BR, Ghori MU. “Global Prevalence and Risk Factors of Gastro-oesophageal Reflux Disease (GORD): Systematic Review with Meta-analysis,” Sci Rep, 2020; 10(1): p. 5814. doi: 10.1038/s41598-020-62795-1.
- Oliveria SA, Christos PJ, Talley NJ, Dannenberg AJ. “Heartburn Risk Factors, Knowledge, and Prevention Strategies: a population-based survey of individuals with heartburn,” Arch Intern Med, 1999; 159(14): p. 1592. doi: 10.1001/archinte.159.14.1592.
- Moayyedi, et al., “The Leeds Dyspepsia Questionnaire: a valid tool for measuring the presence and severity of dyspepsia,” Aliment Pharmacol Ther, 1998; 12(12): pp. 1257–1262. doi: 10.1046/j.1365-2036.1998.00404.x.
- Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. “The importance of patient-reported outcomes in clinical trials and strategies for future optimization,” Patient Relat Outcome Meas, 2018; 9: pp. 353–367. doi: 10.2147/PROM.S156279.
- Richter JE, Peura D, Benjamin SB, Joelsson B, Whipple J. “Efficacy of Omeprazole for the Treatment of Symptomatic Acid Reflux Disease Without Esophagitis,” Arch Intern Med, 2000; 160(12): p. 1810. doi: 10.1001/archinte.160.12.1810.
- Rentz AM, et al. “Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders,” Quality of Life Research, 2004; 13(10): pp. 1737–1749. doi: 10.1007/s11136-004-9567-x.
- De la loge C, et al. “Cross-cultural development and validation of a patient self-administered questionnaire to assess quality of life in upper gastrointestinal disorders: The PAGI-QOL,” Quality of Life Research, 2004; 13(10): pp. 1751–1762. doi: 10.1007/s11136-004-8751-3.
- Atkinson MJ, et al. “Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease,” Health Qual Life Outcomes, 2004; 2(1): p. 12. doi: 10.1186/1477-7525-2-12.
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. “The Montreal Definition and Classification of Gastroesophageal Reflux Disease: A Global Evidence-Based Consensus,” Am J Gastroenterol, 2006; 101(8): pp. 1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x.
- Nagahara A, et al. “A multicentre randomised trial to compare the efficacy of omeprazole versus rabeprazole in early symptom relief in patients with reflux esophagitis,” J Gastroenterol, 2014; 49(12): pp. 1536–1547. doi: 10.1007/s00535-013-0925-8.
- Ndraha S. “Combination of PPI with a prokinetic drug in gastroesophageal reflux disease.,” Acta Med Indones, 2011; 43(4): pp. 233–236.
- Dean BB, Gano AD, Knight K, Ofman JJ, Fass R. “Effectiveness of proton pump inhibitors in nonerosive reflux disease,” Clinical Gastroenterology and Hepatology, 2004; 2(8): pp. 656–664. doi: 10.1016/S1542-3565(04)00288-5.
- Marakhouski K, Karaseva G, Ulasivich D, Marakhouski YK. “Omeprazole-Domperidone Fixed Dose Combination vs Omeprazole Monotherapy: A Phase 4, Open-Label, Comparative, Parallel Randomized Controlled Study in Mild to Moderate Gastroesophageal Reflux Disease,” Clin Med Insights Gastroenterol, 2017; 10: p. 117955221770945. doi: 10.1177/1179552217709456.
- Zamani NF, Sjahid AS, Tuan Kamauzaman TH, Lee YY, Islam MA. “Efficacy and Safety of Domperidone in Combination with Proton Pump Inhibitors in Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis of Randomised Controlled Trials,” J Clin Med, 2022; 11(18): p. 5268. doi: 10.3390/jcm11185268.

