Recent Advances on Structure, Metabolism, and Function of Human Milk Oligosaccharides and the Role in Immunity and Infant Nutrition Enforcement …!
Said Eldeib1,*, Jayaraj Damodaran1, Abdulrahman Zeyada2, Walid Rozik3, Ashraf Elbatal4, Gamal metwallli metwalli5, Sherif Gad6 and Hadi Haddad7
1Dept of paediatrics, ADSCC/Yas clinic Hospital, UAE
2Dept of paediatrics, NMC royal hospital, UAE
3Dept of paediatrics, Amana health care hospital, UAE
4Dept of paediatrics, Salma rehabilitation children Hospital, UAE
5Dept of paediatrics, SSMC, mayo clinic, UAE
6Dept of paediatrics, University hospital Sharjah, UAE
7Dept of paediatrics, Lebanese American university MCRIZK, Lebanon
Received Date: 08/02/2023; Published Date: 01/03/2023
*Corresponding author: Said Eldeib, Paediatrician, Paediatric Department, ADSCC/YAS clinic hospital, Abu Dhabi, UAE
Abstract
Human Milk (HM) is the gold standard for nutrition of new-born infants. Human Milk Oligosaccharides (HMOs) are abundantly present in HM and exert multiple beneficial functions, such as support of the colonization of the gut microbiota, reduction of pathogenic infections and support of immune development. HMO-composition is during lactation continuously adapted by the mother to accommodate the needs of the neonate. Unfortunately, for many valid reasons not all neonates can be fed with HM and are either totally or partly fed with cow-milk derived infant formulas, which do not contain HMOs. These cow-milk formulas are supplemented with non-digestible carbohydrates (NDCs) that have functional effects like that of some HMOs, since production of synthetic HMOs is challenging and still very expensive. However, NDCs cannot substitute all HMO functions. More efficacious NDCs may be developed and customized for specific groups of neonates such as pre-matures and allergy prone infants.
Human milk is finely attuned to the needs of infants supporting optimal growth and overall development. In addition to the nutritional components, it contains important bioactive components, such as enzymes, growth factors, antimicrobial compounds, oligosaccharides, and immunological factors .Emerging evidence suggests that the distinct array of oligosaccharides in human milk provides a variety of physiologic benefits to infants, including the establishment of a balanced gut microbiota .prevention of pathogen adhesion to mucosal surfaces , modulation of the immune response , and potential support to brain development . Currently, most infant formulas do not contain human milk oligosaccharides and their absence may contribute to differences in health outcomes that have been observed between human milk- and formula-fed infants.
Human milk contains a wide range of immunomodulatory factors, including immunoglobulins, human milk oligosaccharides, cytokines, microbiome, innate factors and food antigens. Maternal diet can influence the content of human milk as it is well-established that dietary antigens can be secreted in human milk after maternal consumption, but whether these dietary antigens promote tolerance or sensitization in the infant is a subject of debate.
This review summarizes the current literature on these immunologically active factors in human milk, including the microbiome, innate factors, and maternal diet-derived dietary antigens in the context of infant growth and development of allergic diseases, with the focus on food allergy.
Keywords: Human milk oligosaccharide; Non-digestible carbohydrates prebiotic; Postbiotic; 2′-linked fucosyllactose (2′-FL); 3-galactosyllactose (3′-GL)
Introduction
As per the World Health Organization, infants must be exclusively breastfed during the first six months of life. Human breast milk provides more than half of the child’s nutritional needs during the second year of life [1]. World Health Organization. 2018. The infants who are formula-fed are more prone to infectious diseases, such as gastroenteritis and acute otitis media, and immune-mediated diseases such as allergy, when compared to the infants who are exclusively breastfed [2]. ESPGHAN Committee on Nutrition, Agostoni C, Braegger C2009. The first milk produced by mothers after the delivery is called colostrum, it is biochemically, and functionally different from the mature milk [3]. The human milk is a rich and complete nourishment that is essential for the correct development of the infant’s organism [4]. Colostrum, indeed, contains high concentration of lactoferrin, Immunoglobulin A (IgA), leukocytes and specific developmental factors, and a low amount of lactose, potassium and calcium, underlying its immunological functions rather than nutritional [5,6]
From 5 days to 2 weeks postpartum, there is the production of transitional milk which shares some characteristics of colostrum, although its main function is to support new born at nutritional level [7, 8]. Bacteria located in both colostrum and mature milk can stimulate the anti-inflammatory response, by stimulating the production of specific cytokines, reducing the risk of developing a broad range of inflammatory diseases and preventing the expression of immune mediated pathologies, such as asthma and atopic dermatitis. This mini review discusses the composition of human milk and its biological benefit for infants. Additionally, we also discuss how these beneficial effects can be mimicked if breastfeeding is not possible.
Discussion
Microbiota and the rule in the early new born immunity the specific mechanisms that lead to the formation of the human milk microbiota are still unknown; however, there are different hypothesis that can explain the origin of milk associated bacteria. Indeed, some skin or infant’s oral cavity may become an integral component of the milk microbiota by means of a milk flow back into mammary ducts during lactation [9]. This mechanism may justify the presence of cutaneous and oral bacteria that are recovered in the milk microbiota, such as Streptococcus spp. and Staphylococcus spp [10,11]. Interestingly microorganisms belonging to the maternal, human milk contained also a great number of intestinal bacteria, which may spread from the maternal intestinal environment by a mechanism involving dendritic cells (DCs) and CD18+ cells; these cellular types would be able to capture intestinal microorganisms from the gut lumen and transfer them to lactating mammary glands by means of translocation, which results to be increased during late pregnancy and lactation [9]. Consequently, the milk microbiota can shape the initial intestinal microbiome of new-borns, together with the maternal intestinal and vaginal microorganisms that are ingested by the neonate during the passage through the birth canal [11].
Microbiota
The survival advantage of breastfed infants over non-breastfed infants is known since the 1900s. The stool bacterial composition of breastfed infants was reported to be different from that of the formula-fed infants. Additionally, the presence of an unidentified carbohydrate fraction was also reported in human breast milk. The amount and composition of microbiota vary among women, and during the lactation period. Generally, the total microbiota concentration is higher during the early stages of lactation and decreases within the first three months [12-14]. Xu G, Davis, 2017J Thurl S, Munzert M, 2010. The microbiota content of breast milk after term delivery is higher than that after preterm delivery. The HMO fraction is the third most abundant component in human milk after lactose and lipids, excluding water. The HMO content usually varies between 10–15 grams per liter (g/L) of mature milk (or 1.5–2.3 g/100 kcal, assuming an energy density of human milk of 64 kcal/100 mL) and 20–25 g/L of colostrum [15-17]. Bode L. 2012, Kunz C, Kuntz S 2014 Zivkovic AM, 2011The HMO content in the human breast milk is more abundant than the protein content, which is typically around 10 g/L or 1.5 g/100 kcal.
Health Benefits of the Microbiota:
Several studies have reported the beneficial effects of microbiota that include modification of the intestinal microbiota, anti-adhesive effect against pathogens, modulation of the intestinal epithelial cell response, and development of the immune system. We will discuss each of these effects further
Modulation of intestinal microbiota:
Human Milk Oligosaccharides (HMO) are intrinsic components that affect the gut microbiota by providing an energy source for the beneficial intestinal bacteria. Additionally, HMOs affect the health of the host by serving as a decoy receptor for the opportunistic pathogens in the mucosal surface [18]. Salminen S. 2017 One study reported that none of the selected Enterobacteriaceae strains exhibited growth on a medium containing 2′-FL, 6′-sialyllactose or LNnT as a carbohydrate source. However, several strains were capable of utilizing galacto-oligosaccharides (GOS), maltodextrin, and monosaccharide and disaccharide components of HMOs for their growth [19, 20]. The enriched fecal consortia also did not exhibit growth on a medium containing 2′-FL or 6′-sialyllactose, but exhibited limited growth on a medium containing LNnT [19].
Several in vitro studies have demonstrated that HMOs promote the growth of certain but not all Bifidobacterium [15]. Bode L 2012Bifidobacterium longum subsp. Bifidobacterium infantis exhibit good growth on medium supplemented with HMOs, including 2′-FL, as the sole source of carbohydrate [20]. LoCascio RG, 2007 over time, B. infantis consumes all HMOs including its monosaccharide and disaccharide metabolites [21]. Asakuma S, 2011. The growth of Bifidobacterium bifidum is slower than that of B. infantis in the presence of HMOs. Additionally, certain B. longum strains metabolize fucosylated HMOs [15, 21, 22]. Bode L. 2012 Asakuma S, 2011 Garrido D, 2016, The Bifidobacterium kashiwanohense strain exhibits growth in the presence of 2′-FL and 3′-FL [23-25]. HMOs are a preferred substrate for B. infantis. Other bifidobacteria may reduce the nutrients available for potentially harmful bacteria and limit their growth. Additionally, B. infantis produces shortchain fatty acids (SCFAs), which favor the growth of commensal bacteria and not pathogenic bacteria [23]. Gibson GR, 1994A study reported that among the 24-probiotic strains, only B. longum subsp. B. infantis ATCC 15697 and B. infantis M-63 were able to ferment 3′-sialyllactose, 6′-sialyllactose, 2′-FL, and 3′-FL [24].
When infants are fed with a formula supplemented with 2′-FL and LNnT, they develop a distinctive stool bacterial profile that is more similar to that of the breastfed infants compared to the infants that are fed with a formula not supplemented with prebiotics. The bacterial diversity of infants at the age of 3 months exhibited increased colonization with beneficial bifidobacteria and decreased colonization with pathogenic bacteria [25]. Puccio G2017. Antiadhesive properties HMOs improve the host defense mechanism by strengthening the gut barrier function [26]. Angeloni S2005 the HMO, 2′-FL inhibits Campylobacter jejuni infection and C. jejuni -associated mucosal inflammation [27] Figure 1.
An in vitro study demonstrated that 2′-FL attenuates C. jejuni invasion by 80% and inhibits the release of mucosal pro-inflammatory signals. A study on mouse model revealed that the ingestion of 2′-FL inhibits the C. jejuni colonization by 80%, weight loss by 5%, intestinal inflammation, and induction of inflammatory signaling molecules [28]. Ruiz-Palacios GM, A2003. Prospective study on infants suggested that the beneficial effect of 2′-FL includes a reduction in the number of episodes of C. jejuni -associated diarrhea [29]. Morrow AL, Ruiz-Palacios GM, 2005 LNnT was reported to reduce the abundance of Streptococcus pneumoniae in the lungs of an animal model Idänpään-Heikkilä I, 1997 HMOs may function as a decoy receptor for group B Streptococcus [30, 31].
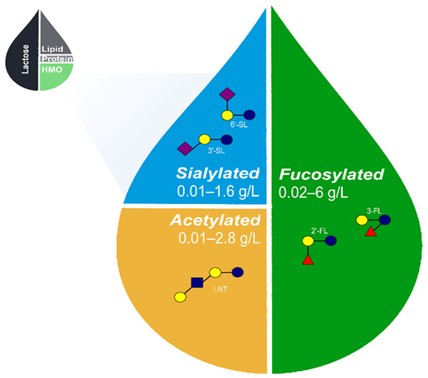
Figure 1: Approximate relative proportions of macronutrients in human breastmilk and range of acetylated, sialylated, and fucosylated HMOs naturally occurring in breastmilk [22,23,57-59,60-62]. The proposed set of 5 core HMOs used in the fortification of infant formula likely recapitulates a wide range of benefits associated with HMOs in human breastmilk. HMO, human milk oligosaccharides; 2′-FL, 2′-fucosyllactose; 3-FL, 3-fucosyllactose; 3′-SL, 3′-sialyllactose; 6′-SL, 6′-sialyllactose; and LNT, lacto-N-tetraose.
HMOs reduce preterm mortality and morbidity by modulating the gut microbiome to protect against necrotizing enterocolitis, candidiasis, and several immune-related diseases [32]. Moukarzel S, 2017LNnT reduces the risk of developing necrotizing enterocolitis in preterm infants [33]. Autran CA 2018Similarly, 2′-FL has also been reported to exhibit beneficial effect against necrotizing enterocolitis [34].
Modulators of intestinal cell response
HMOs can directly affect the intestinal cell response by reducing the cell growth and by inducing differentiation and apoptosis [35]. Kuntz S, Kunz C, 2009 Intestinal health and barrier function are considered the first line of defence in innate immunity. HMOs have been reported to increase the intestinal cell maturation [36]
Immune modulators
One of the important properties of HMOs is the immunomodulation. HMOs directly modulate the gene expression of intestinal cells, leading to changes in the expression of cell surface glycans and other cell responses [37]. Kulinich A, 2016 HMOs modulate lymphocyte cytokine production and enable a more balanced TH1/TH2 response. An increasing number of in vitro. Studies suggest that HMOs exert microbiota-independent effects by directly modulating the immune response and by regulating the immune cell population and cytokine secretion [38]. Donovan SM, 2016HMOs may either act locally on the mucosa-associated lymphoid tissue or act at a systemic level.
The plasma concentration of inflammatory cytokines in the breastfed infants and infants fed with experimental formula supplemented with 2′-FL was markedly lower than that in the infants fed with control formula supplemented with galacto-oligosaccharides [39]. Goehring KC, 2016 these data indicate that infants fed with a formula supplemented with 2′-FL exhibit lower plasma inflammatory cytokine profiles, which is similar to those of a breastfed reference group [39]. Goehring KC, 2016 HMOs were more effective than non-human prebiotic oligosaccharides in modulating the systemic and gastrointestinal immune cell responses in pigs [40]. Comstock SS, 2017 these altered immune cell populations may mediate the rotavirus infection susceptibility [40]. Comstock SS, 2017 the symptoms of food allergy are reduced by 2′-FL through induction of interleukin-10+ T-regulatory cells and through indirect stabilization of mast cells [41].
HMOs, especially 2′-FL, directly inhibit the lipopolysaccharidemediated inflammation during enter toxigenic Escherichia coli invasion of T84 and H4 intestinal epithelial cells through attenuation of CD14 induction [42]. He Y, Liu S, 2016, CD14 expression mediates the lipopolysaccharide-Toll-like receptor 4 stimulation of a part of the macrophage migration inhibitory factors inflammatory pathway by suppressing the cytokine signalling 2/signal transducer and by activating the transcription factor 3/nuclear factor-κB. The direct inhibition of inflammation supports the role of HMOs as a stimulator of the innate immune system [42]. He Y, Liu S, 2016 Two-year-old children who were born through C-section and fed on an infant formula supplemented with 2′-FL had a lower risk of developing immunoglobulin E-associated allergies compared to those fed with unsupplemented formula [43].
Since the early 1980's, research unravelled that breastfed infants show higher fecal numbers of bifidobacterial and a lower faecal pH compared to bottle-fed infants supplemented without prebiotics (44). This lower pH of the stool in breastfed infants is caused by higher lactate and acetate levels (45, ). Infant formula supplemented with a mixture of GOS/FOS promote a more bifidogenic microbiota composition (46) and a SCFA profile more similar to breast-fed infants (47). In line with these findings, our batch cultures showed that GOS induced the outgrowth of bifidobacteria and increased production of lactate. Although to a lesser extent, SL also boosted the abundance of bifidobacteria, which is in line with earlier reports showing that different strains of bifidobacteria are capable of metabolizing both neutral and acidic HMO
New insight into the microbial ecology
The study of microbial interactions within a bacterial population is of extreme importance to clearly understand the specific role of microbiome. Indeed, microorganisms compete for nutrients, exchange genetic material and metabolites, being responsible of influencing the microbiota composition and the host’s health [48]. Due to its dynamic nature and high heterogeneity, the microbiota can be considered a complex and variable ecosystem not often well understandable. For this reason, in the last years a novel approach has been developed to study the microbiota, by using graph theoretical, systems-oriented method able to facilitate the understanding of evolutionary and complex ecological processes [48]. Bacterial network is becoming essential to study microbial relationships and clarify the impact of various interactions on the host by identifying the main “hubs” that may represent the most influential member in a bacterial community [48]. Moreover, a central node is thought to have more links with other hubs, having a pivotal role in the stability of the whole microbial network.
Breast milk, with its complex and dynamic composition, has deep positive impacts on the health and development of new-borns and infants, with great long-term benefits for children and adults. In this paper, we discussed the several physiological and protective roles of HMOs, such as brain and intestinal development or protection from infections. This is one of the miracles of human milk: a single class of molecules has such a great impact on the development of the infant.
This Mini Review is to elucidate the specific immunologic role of the microbiota and its impact on the new-born’s health and life, highlighting the importance to properly study the biological interactions in a bacterial population and between the microbiota and the host. Microbiota can serve as soluble decoy receptors that block the attachment of viral, bacterial, or protozoan parasitic pathogens to the epithelial cell surface receptors, which may aid in preventing infectious diseases. HMOs are also antimicrobials that act as bacteriostatic or bactericidal agents. Additionally, microbiota enhance host epithelial and immune cell responses in the neonate. However, further studies are needed to highlight the direct and strong connection between the human milk microbiota and the stimulation of new-borns’ immune system, as to date there are no clear and specific evidence about this association. Application of network biology will significantly improve our knowledge on bacterial interactions among the milk microbiota, with important applications for eventual targeted modification of bacterial composition, aimed to enhance the abundance of those microorganisms that may be essential not only for the modulation of the infants’ immune system, but also for improving the whole host’s health.
Human milk oligosaccharides may also influence the infants on a systemic level. Obviously, they are partially absorbed in the intestine of babies, and can be detected in the urine of breast-fed infants). Some evidence exists that milk oligosaccharides may function as anti-inflammatory factors, contributing to the lower incidence and severity of inflammatory diseases in breast-fed infants). Particularly, sialic acid-containing oligosaccharides were found to inhibit the formation of platelet–neutrophil complexes and neutrophil activation. In addition, the acidic oligosaccharide fraction significantly inhibited leucocyte rolling and adhesion on epithelial cells.
In healthy infants consuming a partly fermented infant formula (IF) with postbiotics, 2′-linked fucosyllactose (2′-FL), a specific prebiotic mixture of short-chain galacto-oligosaccharides (scGOS) and long-chain fructo-oligosaccharides (lcFOS), and milk fat. This double-blind, controlled trial randomised 215 fully IF-fed infants ≤ 14 days of age to either: Test Group (IF) containing 26% fermented formula with postbiotics derived from Lactofidus fermentation process (including 3′-Galactosyllactose; 3′-GL), 0.8 g/100 mL scGOS/lcFOS (9:1), 0.1 g/100 mL 2′-FL, and milk fat), or Control group (IF with 0.8 g/100 mL scGOS/lcFOS (9:1)) until 17 weeks of age.
Fully breastfed infants were included as a reference. Anthropometric measures, gastrointestinal symptoms, and safety were assessed monthly. Equivalence in weight gain (primary outcome) between the Test and Control groups was confirmed (difference in means −0.08 g/day; 90% CI (−1.47;1.31)) with estimated mean weight gain (SE) of 31.00 (0.59) g/day and 31.08 (0.60) g/day, respectively, (PP population, n = 196).
Equivalence in length and head circumference gain between the randomised groups was also confirmed. No statistically significant differences were observed in adverse events or gastrointestinal tolerance between randomised IF groups. A partly fermented IF with postbiotics, specific oligosaccharides, 2′-FL, and milk fat supports adequate infant growth and is safe and well-tolerated in healthy term infants.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Data Availability Statement: Not applicable.
Funding: This research received no external funding.
Conflicts of Interest: All other authors report no conflicts of interest. Data Availability Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
References
- World Health Organization. Maternal, newborn, child and adolescent health, 2018.
- Agostoni C, Braegger C, Desir T, Kolacek S, et al. ESPGHAN Committee on Nutrition, Breast-feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr, 2009; 49: 112-125.
- Castellote C, Casillas R, Ramírez-Santana C, Pérez-Cano FJ, Castell M, et al. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J Nutr, 2011; 141: 1181-1187.
- Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am, 2013; 60: 49-74.
- Kulski JK, Hartmann PE. Changes in human milk composition during the initiation of lactation. Aust J Exp Biol Med Sci, 1981; 59: 101-114.
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia, 2007; 12: 211-221.
- Henderson JJ, Hartmann PE, Newnham JP, Simmer K. Effect of preterm birth and antenatal corticosteroid treatment on lacto genesis II in women. Pediatrics, 2008; 121: e92-e100.
- Nommsen-Rivers LA, Dolan LM, Huang B. Timing of stage II lacto genesis is predicted by antenatal metabolic health in a cohort of primiparas. Breastfeed Med, 2012; 7: 43-49.
- Rodríguez JM. The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv Nutr, 2014; 5: 779-784.
- Gao Z, Tseng C, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci USA, 2007; 104: 2927-2932.
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, et al. Topographical and temporal diversity of the human skin microbiome. Science, 2009; 324: 1190-1192.
- Houghteling PD, Walker WA. Why is initial bacterial colonization of the intestine important to infants’ and children’s health? J Pediatr Gastroenterol Nutr, 2015; 60: 294-307.
- Xu G, Davis JC, Goonatilleke E, Smilowitz JT, German JB, et al. Absolute quantitation of human milk oligosaccharides reveals phenotypic variations during lactation. J Nutr, 2017; 147: 117-124.
- Thurl S, Munzert M, Henker J, Boehm G, Müller-Werner B, et al. Variation of human milk oligosaccharides in relation to milk groups and locational periods. Br J Nutr, 2010; 104: 1261-1271.
- Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology, 2012; 22: 1147-1162.
- Kunz C, Kuntz S, Rudloff S. Bioactivity of human milk oligosaccharides. In: Moreno FM, Sanz ML, eds. Food Oligosaccharides: Production, Analysis and Bioactivity. 1st ed. Chichester: John Wiley & Sons, Ltd 2014: 5-20.
- Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci USA, 2011; 108: 4653-4658.
- Salminen S. Regulatory aspect of human milk oligosaccharides. Nestle Nutr Inst Workshop Ser, 2017; 88: 161-170.
- Hoeflinger JL, Davis SR, Chow J, Miller MJ. In vitro impact of human milk oligosaccharides on Enterobacteriaceae growth. J Agric Food Chem, 2015; 63: 3295-3302.
- LoCascio RG, Ninonuevo MR, Freeman SL, Sela DA, Grimm R, et al. Glycol profiling of bifid bacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J Agric Food Chem, 2007; 55: 8914-8919.
- Asakuma S, Hatakeyama E, Urashima T, Yoshida E, Katayama T, et al. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J Biol Chem, 2011; 286: 34583-34592.
- Garrido D, Ruiz-Moyano S, Kirmiz N, Davis JC, Totten SM, et al. A novel gene cluster allows preferential utilization of fucosylated milk oligosaccharides in Bifidobacterium longum subsp. longum SC596. Sci Rep, 2016; 6: 35045.
- Gibson GR, Wang X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol, 1994; 77: 412-420.
- Thongaram T, Hoeflinger JL, Chow J, Miller MJ. Human milk oligosaccharide consumption by probiotic and humanassociated bifidobacteria and lactobacilli. J Dairy Sci, 2017; 100: 7825-7833.
- Puccio G, Alliet P, Cajozzo C, Janssens E, Corsello G, et al. Effects of infant formula with human milk oligosaccharides on growth and morbidity: a randomized multicenter trial. J Pediatr Gastroenterol Nutr, 2017; 64: 624-631.
- Angeloni S, Ridet JL, Kusy N, Gao H, Crevoisier F, et al. Glyco profiling with micro-arrays of glycoconjugates and lectins. Glycobiology, 2005; 15: 31-41.
- Yu ZT, Nanthakumar NN, Newburg DS. The human milk oligosaccharide 2′-fucosyllactose quenches Campylobacter jejuni -induced inflammation in human epithelial cells HEp-2 and HT-29 and in mouse intestinal mucosa. J Nutr, 2016; 146: 1980- 1990.
- Morrow AL, Ruiz-Palacios GM, Jiang X, Newburg DS. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J Nutr, 2005; 135: 1304-1307.
- Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fucα1, 2Galβ1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem, 2003; 278: 14112-14020.
- Idänpään-Heikkilä I, Simon PM, Zopf D, Vullo T, Cahill P, et al. Oligosaccharides interfere with the establishment and progression of experimental pneumococcal pneumonia. J Infect Dis, 1997; 176: 704-712.
- Lin AE, Autran CA, Szyszka A, Escajadillo T, Huang M, et al. Human milk oligosaccharides inhibit growth of group B Streptococcus. J Biol Chem, 2017; 292: 11243-11249.
- Moukarzel S, Bode L. Human milk oligosaccharides and the preterm infant: a journey in sickness and in health. Clin Perinatol, 2017; 44: 193-207.
- Autran CA, Kellman BP, Kim JH, Asztalos E, Blood AB, et al. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut, 2018; 67: 1064-1070.
- Good M, Sodhi CP, Yamaguchi Y, Jia H, Lu P, et al. The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br J Nutr, 2016; 116: 1175-1187.
- Kuntz S, Kunz C, Rudloff S. Oligosaccharides from human milk induce growth arrest via G2/M by influencing growth-related cell cycle genes in intestinal epithelial cells. Br J Nutr, 2009; 101: 1306-1315.
- Holscher HD, Davis SR, Tappenden KA. Human milk oligosaccharides influence maturation of human intestinal Caco2Bbe and HT-29 cell lines. J Nutr, 2014;144: 586-591.
- Kulinich A, Liu L. Human milk oligosaccharides: The role in the fine-tuning of innate immune responses. Carbohydr Res, 2016; 432: 62-70.
- Donovan SM, Comstock SS. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann Nutr Metab, 2016; 69: 42-51.
- Goehring KC, Marriage BJ, Oliver JS, Wilder JA, Barrett EG, et al. Similar to those who are breastfed, infants fed a formula containing 2′-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J Nutr, 2016; 146: 2559- 2566.
- Comstock SS, Li M, Wang M, Monaco MH, Kuhlenschmidt TB, et al. Dietary human milk oligosaccharides but not prebiotic oligosaccharides increase circulating natural killer cell and mesenteric lymph node memory T cell populations in no infected and rotavirus-infected neonatal piglets. J Nutr, 2017; 147: 1041-1047.
- Castillo-Courtade L, Han S, Lee S, Mian FM, Buck R, et al. Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy, 2015; 70: 1091-1102.
- He Y, Liu S, Kling DE, Leone S, Lawlor NT, et al. The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut, 2016; 65: 33-46.
- Sprenger N, Lee LY, De Castro CA, Steenhout P, Thakkar SK. Longitudinal change of selected human milk oligosaccharides and association to infants’ growth, an observatory, single center, longitudinal cohort study. PLoS One, 2017; 12: e0171814.
- Matsuki T, Yahagi K, Mori H, Matsumoto H, Hara T, Tajima S, et al. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat Commun, 2016; 7: 1–12. doi: 10.1038/ncomms11939
- Braegger C, Chmielewska A, Decsi T, Kolacek S, Mihatsch W, Moreno L, et al. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr, 2011; 52: 238–250. doi: 10.1097/MPG.0b013e3181fb9e80
- Moro G, Minoli I, Mosca M, Fanaro S, Jelinek J, Stahl B, et al. Dosage-related bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants. J Pediatr Gastroenterol Nutr, 2002; 34: 291–295. doi: 10.1097/00005176-200203000-00014
- Bode L, Jantscher-krenn E. Structure-function relationships of human milk oligosaccharides. Adv Nutr An Int Rev J,2012; 3: 383S–391S. 10.3945/an.111.001404.
- Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients, 2017; 9(9): 1021.

