Study on the Impact of Public Health Measures on the Vaccine Phase Ⅲ Clinical Trial Period
Ruyun Wang*
Key Laboratory of Marine Hazards Forecasting, Ministry of Natural Resources (MNR), Hohai University, Nanjing, China.
College of Oceanography, Hohai University, Nanjing, China.
Received Date: 24/02/2022; Published Date: 08/03/2022
*Corresponding author: Ruyun Wang*
Abstract
We derived the ratio of the period of time required for phase Ⅲ trials for all vaccines in areas with and without public health interventions. The ratio is directly controlled by the public health protection rate, the greater the public health protection rate, the greater the proportion; When the public health protection rate tends to 1, this ratio tends to infinity.
Keywords: Public health measures; Vaccine; Phase Ⅲ clinical trial; Trial period
Introduction
Phase Ⅲ vaccine trials typically involve double-blind trials that divide large groups of people into control and vaccination groups. Unblinding was performed when the number of patients in the control group and vaccine group reached the required number of statistics. The incidence rates of the control and vaccination groups were calculated, as well as the current vaccine protection rate1. It often takes years to meet the unblinding requirements for phase Ⅲ vaccine trials, which is costly and risky for r&d organizations. Wang Ruyun (2021) studied the intermediate unblinding conditions and protection rate t public health measures. The ratio is directly controlled by the public health protection rate, the greater the public health protection rate, the greater the proportion; When the public health protection rate tends to 1, this ratio tends to infinity. Thus, the internal influence of health protection requirements is not high if unblinding requirements are to be met as quickly as possible. At the same time, it also reminds us that there may be problems with the currently used indicators for evaluating the effectiveness of vaccine protection and the eligibility criteria for testing vaccines.
Vaccine-related protection rate and evaluation criteria of qualified vaccines
Assume that the number of participants in the phase Ⅲ vaccine trial is m in both the control and vaccination groups.
In areas where no public health measures were taken, the number of cases in the control group and the vaccination group were respectively marked as mc/n, mv/n, and the incidence of cases in the control group and the vaccination group was respectively
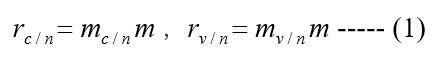
The corresponding rate bv/n measures have been adopted is of vaccine protection in areas where no public health
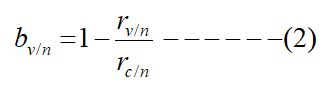
In areas where public health measures were taken, the number of cases in the control group and the vaccination group were recorded as mc/p, mv/p and the incidence of cases in the control group and the vaccination group were respectively

The corresponding rate bv/p measures is of vaccine protection in areas with public health

Suppose that the incidence of disease in a region without public health measures is rn, and the incidence of disease after public health measures is rp, then the protection rate
bp of public health measures is
According to the World Health Organization, the qualifying standard for vaccine protection is
The impact of public health measures on the phase iii trial period of vaccines
Since phase III vaccine trials are usually unblinded when a set statistically significant number m0 of infected persons has been reached, the unblinding period required for the trials in areas where no public health measures were adopted and where public health measures were adopted were Tn, Tp, respectively, and the time unit and the time period unit of incidence were both months.
The following studies were based on the assumption that the incidence would be the same in the absence of public health measures in different areas of the phase 3 vaccine trials.
Can be obtained by formula (1)-(4)


Table 1: Tp/Tn under different public health measures.
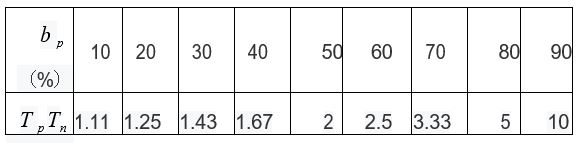
As can be seen from Table 1, phase Ⅲ vaccine trials in areas with better public health practices require longer unblinding times than those in areas with no public health practices. Assuming that the unblinding time required in a region with no public health interventions were 3 months, the phase 3 trial in bp = 80 % would take 15 months.
By comparing the left side of Formula (11) and (12), it can be found that due to the intervention of public health measures, the number of cases decreased by a factor (1 - bp) of two in the same period, and the larger bp, the more the decrease, so it is necessary to increase the trial period.
In addition, it can be seen from the left end of formula (11) and (12) containing factors 2 - bv/n and 2 - bv/p, the larger bv/n, bv/p, the lower the number of patients, thus increasing the time of phase 3 trial. The more protective the vaccine, the longer the trial period. However, due to 1 £ 2 - bv / n £ 2 and 1 £ 2 - bv / p £ 2, the effect on the trial period is limited, which is at most twice the unblinding time required in the case of complete vaccine failure.
References:
- Halloran ME, Longini IM, Struchiner CJ. Design and Analysis of Vaccine Studies. Springer Verlag, 2010. ISBN 978-0-387-40313-7.
- Ruyun Wang. Study on Uncovering Blindness Requirements and Protection Rate in the Midterm of Phase Ⅲ Clinical Trial of COVID-19 Vaccine. Advances in Applied Mathematics, 2021; 10(3): 781-789.

