Nasal Irrigation in the COVID-19 Era
Stella Georgiou and Konstantinos Alevizopoulos*
Gerolymatos International S.A., Research & Development Department, Gerolymatos International S.A. Kryoneri, Greece
Received Date: 07/09/2021; Published Date: 29/09/2021
*Corresponding author: Konstantinos Alevizopoulos PhD, Gerolymatos International S.A., Research & Development Department, Gerolymatos International S.A., Kryoneri, Address: 13 Asklipiou Str. 14568, Kryoneri, Attica, Greece.
Abstract
Rapid spread of SARS-CoV-2 to the community leading to the COVID-19 disease has undoubtedly changed individuals’ lives and attitudes and has threatened healthcare systems globally. SARS-CoV-2 attaches and initiates its lifecycle in the nasopharyngeal mucosa via infected droplet inhalation which leads to high viral loads and increased disease transmission and severity. Other than preventive vaccination, additional measures to mitigate both the transmission and progression of SARS-CoV-2 are currently sought. Nasal irrigation can prevent early transmission, minimize viral shedding and disease severity, and limit complications by mechanical washing-out of infectious particles present in the nasal cavities. Several publications have now proposed that both isotonic (0.9% NaCl) and hypertonic (>0.9% NaCl) solutions can be utilized for nasal lavage in COVID-19 patients. Considering that nasal irrigations are safe and easy to use, this nonpharmacological remedy could be adopted by the public, patients and healthcare workers in early disease stages. In this review, the use of nasal rinsing as an adjunctive prophylactic measure to defend against SARS-CoV-2 is summarized and advocated.
Keywords: Nasal irrigation; Isotonic solution; Hypertonic solution; SARS-CoV-2; COVID-19; Viral load reduction
Mini Review
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which causes coronavirus disease-2019 (COVID-19), has brought us against an unprecedented global health crisis with the death toll increasing dramatically each passing day. Since the first outbreak in Wuhan, there have been more than 217 million confirmed cases of COVID-19, including more than 4.5 million deaths according to WHO (https://covid19.who.int/). The disease develops initially as a respiratory infection. Nasal, conjunctival and oral mucosa are entry portals of SARS-CoV-2 as the infection is mainly transmitted by exposure to infected respiratory droplets. The nasal epithelium and nasopharyngeal mucosa are also sites of replication of SARS-CoV-2 with nasal swabs presenting higher viral load compared to throat swabs [1]. The high transmission rate, the multiple SARS-CoV-2 variants and the lack of targeted treatments to combat the pandemic apart from available vaccines, render the addition of extra preventive measures of paramount importance.
Nasal Irrigation (NI) is a well-established practice that has been used for centuries to clean the airways. It facilitates physical drainage of thick mucus, allergens, infective debris and inflammatory mediators while enhancing hydration of the nasal mucosa lining and mucociliary function [2]. Both isotonic (0.9% NaCl) and hypertonic (>0.9% NaCl) solutions are used for nasal douching, with hypertonic solutions yielding more beneficial effects improving nasal symptoms such as nasal congestion, sneezing, nasal itching and rhinorrhea [3]. Recent in vitro studies suggest that solutions rich in chloride ions, e.g., hypertonic solutions with high NaCl strength, may exert anti-viral effects. In these studies, the influx of Cl− ions generated by NaCl was found to block replication of HSV-1, RSV, MHV68, and CV-B3 viruses in the Vero cell line. More importantly, HCoV-229E (human coronavirus 229E) was significantly inhibited from concentrations of 30mM NaCl and above [4]. Similar results were obtained by Machado [5] who showed that 260 mM NaCl (corresponding to 1.5% NaCl) inhibit 100% SARS-CoV-2 replication in the same cells. Inhibition of virus replication was attributed to an intracellular mechanism and not due to the interaction of SARS-CoV-2 spike protein with its human receptor ACE2 that is indispensable for viral infection. Finally, a third in vitro study showed that the proliferation of yeast on the surface of a facepiece may strongly be reduced when this is coated with hypertonic saline. The authors proposed that this unspecific inhibitory salt effect could protect mask carriers efficiently from SARS-CoV-2 infections [6]. Preclinical studies supporting use of hypertonic solutions for SARS-CoV-2 inhibition can be found in Table 1.
Table 1: Preclinical studies supporting use of hypertonic solutions for SARS-CoV-2 inhibition.
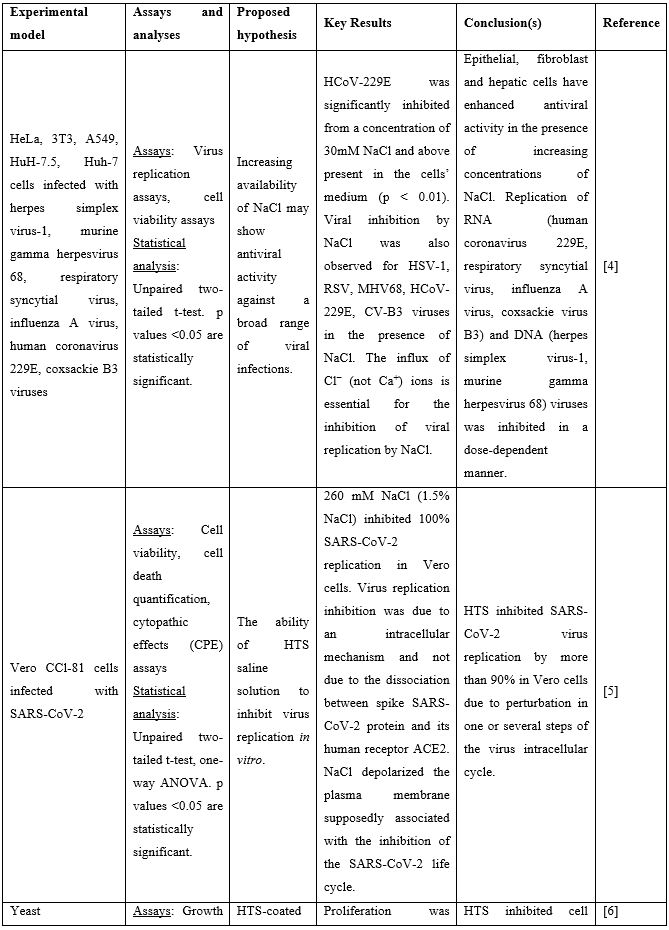
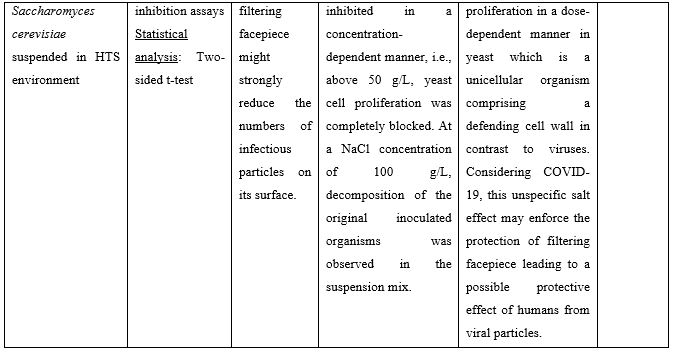
Abbreviations: ACE2: Angiotensin Converting Enzyme 2; COVID-19: Corona Virus Disease 19; CPE: Cytopathic Effect; HCoV: Human Corona Virus; HTS: Hypertonic Solution; NaCl: Sodium Chloride; SARS-CoV-2: Severe Acute Respiratory Syndrome Corona Virus.
Given the high viral titers detected in the upper respiratory tract of asymptomatic and symptomatic individuals [1], and in agreement with the preclinical data mentioned above, use of NI as a means to bring down the viral load, reduce transmission, symptoms and duration of illness has been proposed recently in a series of publications. In human samples, Ramalingam and colleagues [7] were the first to show reduced viral shedding as a result of use of hypertonic saline nasal irrigation (HSNI; 2-3% NaCl) combined with standard treatment versus standard treatment alone in the ELVIS (Edinburgh and Lothians Viral Intervention Study) clinical study. The study participants suffered from common cold originating from several viruses including Rhinovirus, coronavirus COV-229E and other strains, Influenza A virus, and Human metapneumovirus. In a follow up study [8] conducted by the same investigators, post-hoc secondary analysis of data from the ELVIS study showed that hypertonic saline nasal irrigation and gargling (HSNIG) reduced the duration of coronavirus upper respiratory tract infection (URTI) by an average of two-and-a-half days. Overall, NI with hypertonic solution reduced illness duration by 22%, use of over-the-counter medication for symptom relief by 36%, and virus spread to the family by 35%. These conclusions suggested that saline rinses can reduce symptom burden and decrease viral shedding -including that of coronaviruses- in patients.
A series of additional publications confirmed the results mentioned above further promoting NI as an easily implementable technique to be used for preventing and/or controlling COVID-19 infection. Chatterjee et al [9] performed a clinical trial to investigate if normal saline nasal spray and gargle (NSNSG) can protect nasopharyngeal mucosa from SARS-CoV-2 infection. 129 individuals with laboratory confirmed SARS-CoV-2 were allocated to study and control groups. In the study group, 61 patients received NSNSG for 4 days whereas 68 patients in the control group received no intervention. In 91% of patients of the study group, SARS-CoV-2 severity score was either improved or did not progress compare to the control group. Tolerability with NSNSG was found acceptable in the study group. The authors concluded that NSNSG significantly washes off SARS-CoV-2 from the nasal cavity, pharynx and the lung alveoli preventing micro aspiration and pneumonia [9]. NSNSG with hypertonic solution performed several times a day also helped a 54-year-old asymptomatic SARS-CoV-2 infected physician who was quarantined. The infected physician remained asymptomatic, and serum immunoglobulin IgG for SARS-CoV-2 was tested negative two months after the infection [10].
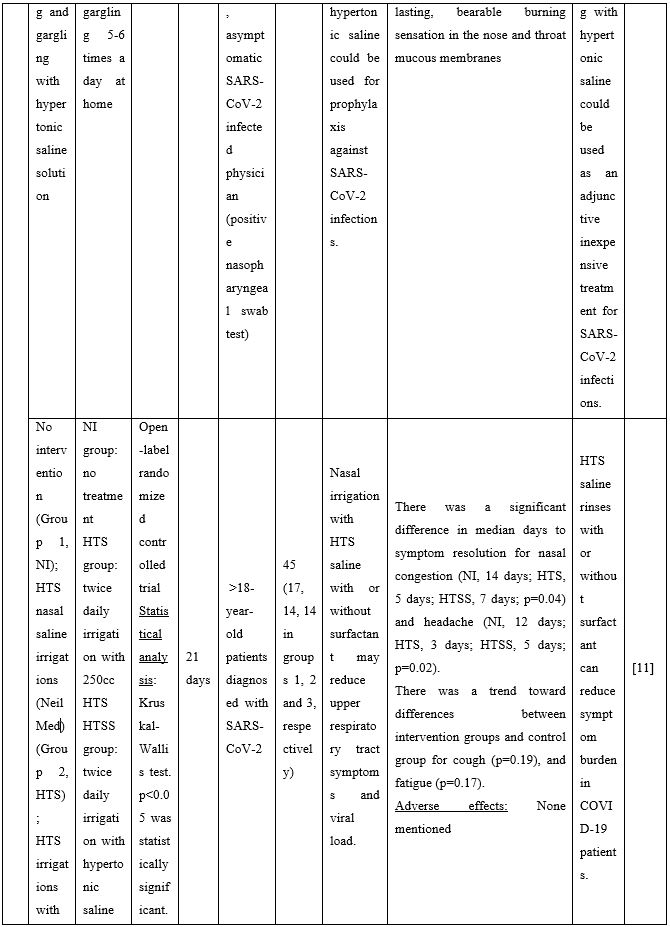
In non-hospitalized patients with COVID-19, hypertonic saline rinses can reduce symptom burden. An open-label randomized controlled trial evaluated the effect of NI with hypertonic saline or saline with 1% surfactant on upper respiratory symptoms and viral load. Analysis of nasal swabs showed that NI relieved upper respiratory symptoms with nasal congestion and headache resolving a median of 7-9 days earlier in the intervention groups. Additionally, headache was significantly reduced. A trend toward decreased cough and fatigue between groups was also reported [11]. Another study found that when COVID-19 patients performed HSNI supplemented with steroids, the olfactory dysfunction duration was significantly reduced restoring the sense of smell [12].
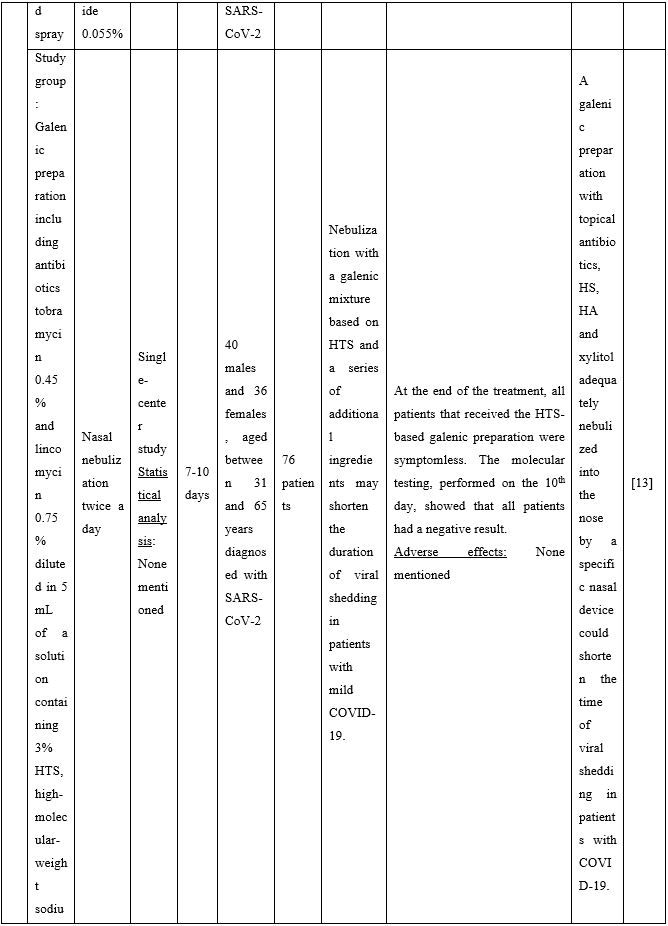
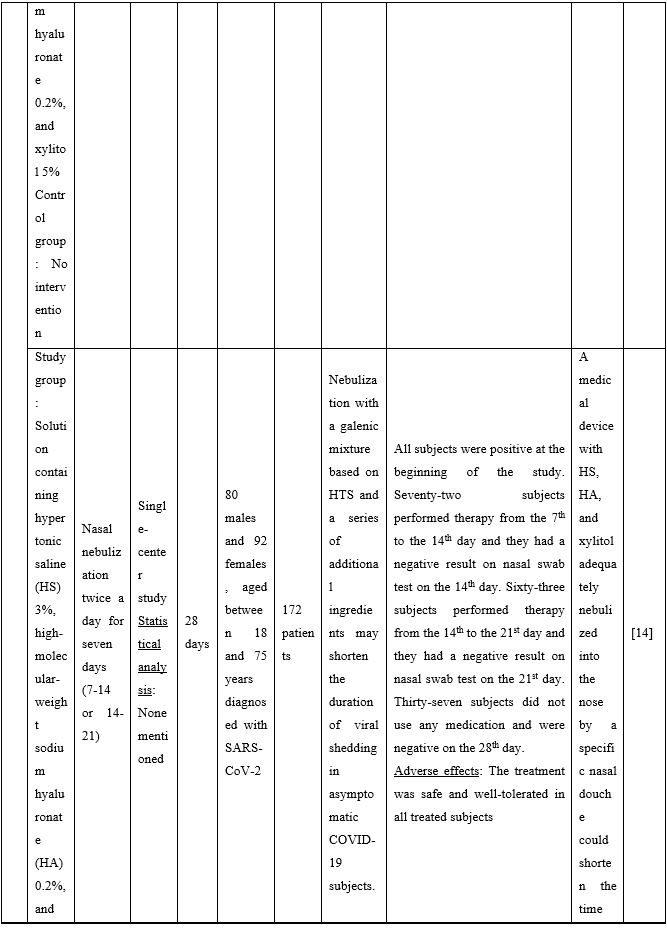
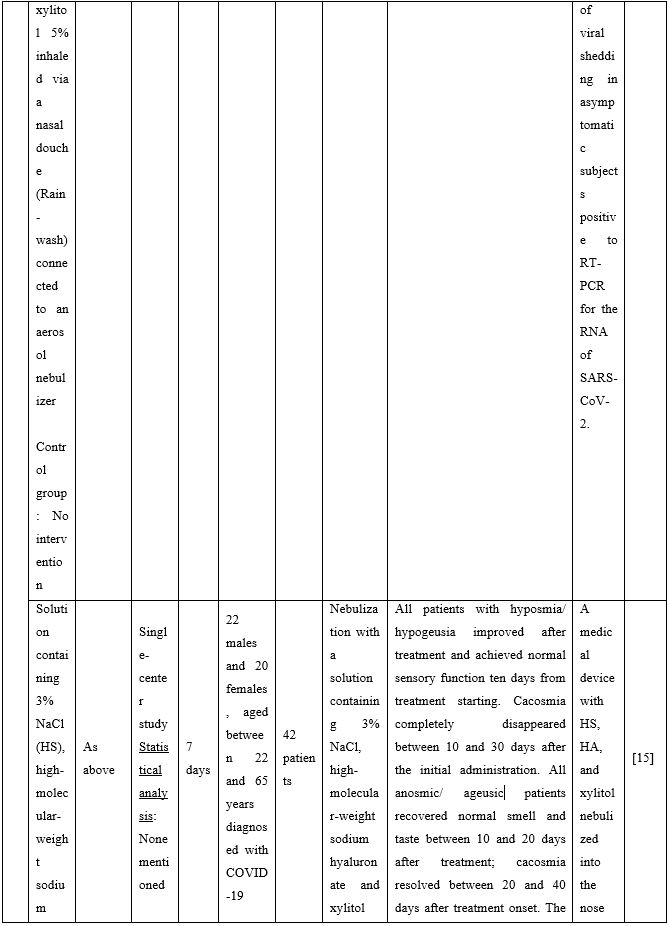
Duration of viral shedding of SARS-CoV-2 after nebulization with a galenic mixture based on HTS was reduced in two different clinical studies comprising 248 patients with mild or asymptomatic COVID-19. In the first study including 76 patients with mild disease phenotype, a galenic preparation containing hypertonic saline (3%), high-molecular-weight sodium hyaluronate, xylitol and antibiotics was administered twice daily for a period of up to 10 days. At the end of the treatment, all patients were symptomless and had a negative molecular test on the 10th day [13]. In the second study, 172 asymptomatic COVID-19 subjects received the HTS-based preparation mentioned above without antibiotics for 7 days; the patients were negative at the nasal swab testing performed on the 14th or 21st post-treatment day [14]. The same hypertonic solution was additionally administered via nebulization in 42 patients with hyposmia/hypogeusia due to COVID-19. All patients in the study improved after treatment and achieved normal sensory function ten days after treatment initiation. Cacosmia completely disappeared between 10- and 30-days post-treatment [15]. In all cases, the treatment was safe and well-tolerated in all treated subjects. The results from infected patients with mild or no symptoms showed that NI with hypertonic solutions represents a simple, practically feasible, and globally implementable strategy with important therapeutic value. All research studies supporting use of hypertonic or isotonic solutions in patients with COVID-19 can be found in Table 2.
Table 2. Clinical studies and case reports supporting use of hypertonic or isotonic solutions in patients with COVID-19.
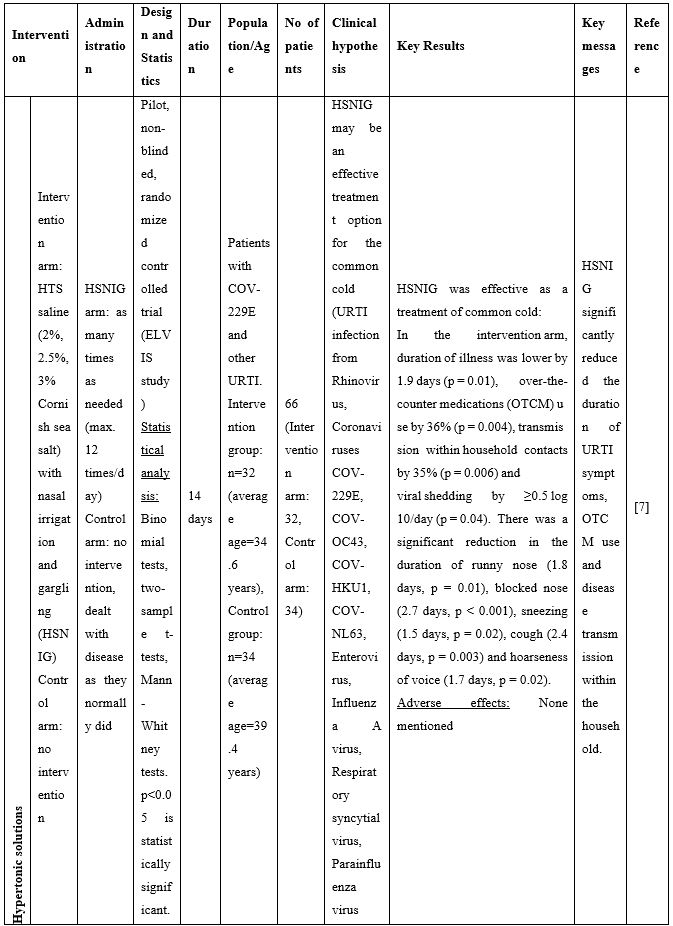
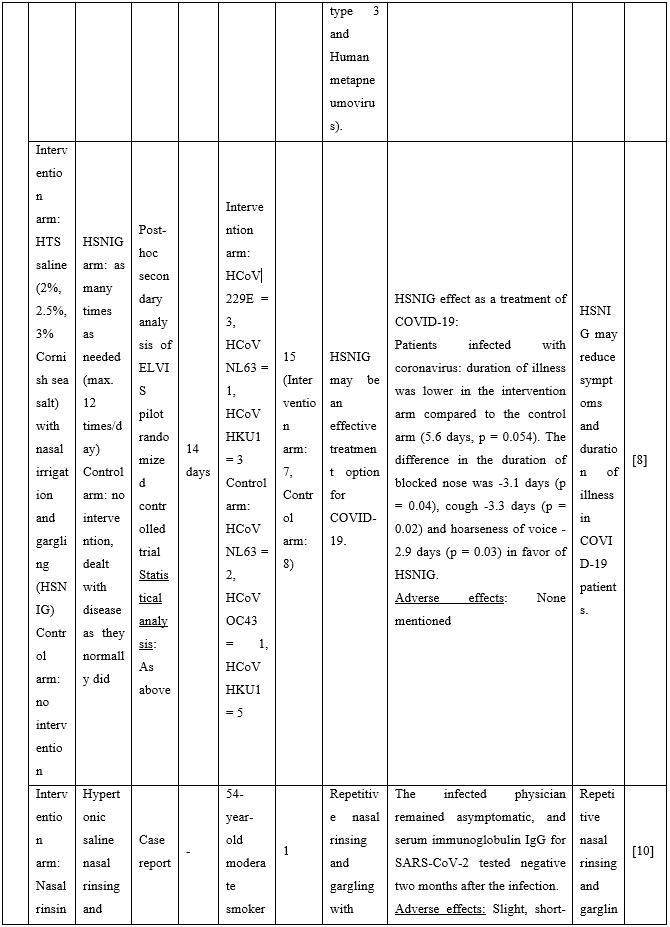

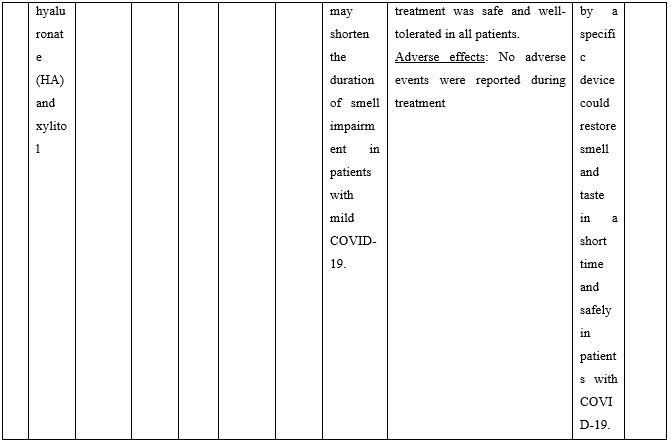
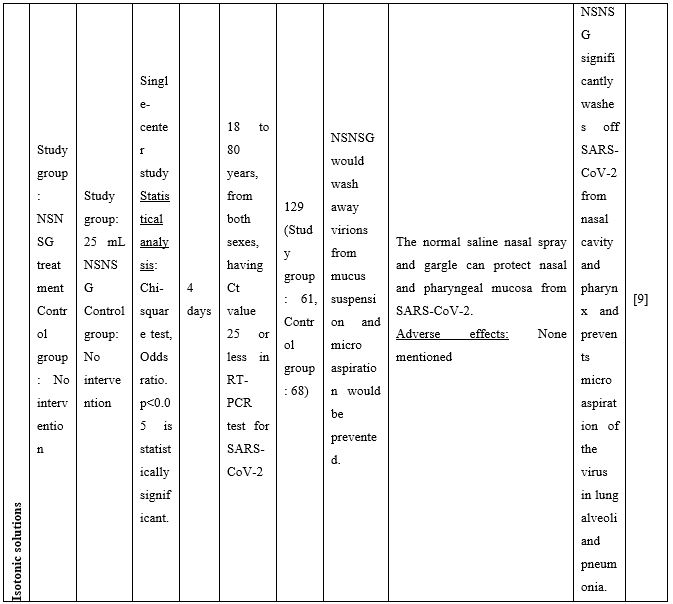
Abbreviations:
COVID-19: Corona Virus Disease 19; HA: Sodium Hyaluronate; HS: Hypertonic Saline; HCoV: Human Corona Virus; HSNIG: Hypertonic Solution with Nasal Irrigation and Gargling; HTS: Hypertonic Solution; HTSS: Hypertonic Solution plus Surfactant; NaCl: Sodium Chloride; NI: No Intervention; NSNSG: Normal Saline Nasal Spray and Gargle; OTCM: Over-the-counter Medication; SARS-CoV-2: Severe Acute Respiratory Syndrome Corona Virus; URTI: Upper Respiratory Tract Infections.
In agreement with the experimental conclusions reached above, several review studies strongly support use of NI to reduce symptom burden and break the chain of infection. For example, Singh et al [16] drew a parallel between nasal irrigation and hand washing used to contain the spread of the infection. These interventions may be useful in reducing nasopharyngeal viral titers and viral shedding, ultimately leading to COVID-19 transmission reduction. Shetty and co-workers [17] drafted a guide for use of topical solutions for the prophylaxis of healthcare professionals dealing with COVID-19 infected patients. It was noted that irrigation of the nasopharynx and gargle of the oropharynx with hypertonic saline, twice daily before and after patient exposure, could decrease viral attachment and entry into cells. Similar conclusions were reached by a hypothesis paper by Panta and co-workers [18]. Farrell and colleagues [19] postulated that saline irrigations with or without indicated additives are safe to use in the presence of COVID-19 especially for patients who already use these therapies for rhinosinusitis management. Stathis and co-workers [20] compared several commonly used nasal antiseptics and gargles including irrigations with hypertonic solutions. They concluded that hypertonic solution administered as mouth wash and/or NI can be used for reducing the rate of transmission, for reducing viral load very early after infection and for prophylaxis after exposure to SARS-CoV-2. Daily nasal douching and gargling combining hypertonic saline solution with virucidal mucosal therapies such as 0.23% aqueous PVP iodine has also been advised for enhanced protection [21].
Having recognized the potential usefulness of nasal douching in COVID-19 management, this technique is now clinically recommended by different medical associations and committees including the French Association of Pediatric Otorhinolaryngology (AFOP) and French Society of Otorhinolaryngology (SFORL), and ENT Pediatric otolaryngology. Ιt was noted that NI with saline can provide a nonpharmacological technique for treating nasal congestion, especially in infants. The same guideline stresses the importance of extra precautions to be implemented in healthcare settings to avoid contamination of a staff caregiver. Even in the absence of NI, the risk of transmitting the virus within the household members is very high because an infected child has a high risk of contaminating his/her family members [22]. Along the same lines, the recommendations by French Rhinology Association (AFR), French ENT College, French ENT National Union (SNORL), and French National Professional ENT Council (CNPORL) provided the framework to protect healthcare workers against COVID-19 while they continued to provide emergency care helping patients. Hand-washing before and after treatment of patients, washing and disinfecting equipment and soiled surfaces weekly, and discarding all the liquid after each nasal wash are among strict hygiene measures should ideally be taken during treatment [23]. Considering acceptance of such practices by health care professionals, NI with hypertonic saline solutions has also been positively judged and accepted by 43.9% of pharmacists attending the 7th National Hospital and Institution Pharmacists Congress. Analysis of 237 questionnaires using the Turkish COVID-19 Scientific Committee guideline highlighted the use of media tools affecting the attitudes of both the public and health professionals towards use of saline irrigations for prevention and control of the spread of the COVID-19 disease [24].
Conclusively, the aforementioned information in the field of COVID-19 in the last months fully supports the use of NI for management of COVID-19 respiratory tract symptoms and for reduction of disease duration in infected patients. The use of hypertonic solutions could confer added clinical benefit compared to isotonic solutions facilitating nasal decongestion and cleansing simultaneously. NI as a cost-free modality can be globally implemented as a prophylactic and therapeutic strategy posing no side effects or complications in the battle against SARS-CoV-2. Additional clinical and epidemiological studies are indispensable to strengthen the results and the inferred recommendations.
Conflicts of Interest/ Competing Interests: No conflict of interest was declared by the authors.
Grant Information: The author(s) received no specific funding for this work.
References
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020; 382(12): 1177-1179. doi: 10.1056/NEJMc2001737. Epub 2020 Feb 19. PMID: 32074444; PMCID: PMC7121626.
- Huijghebaert S, Hoste L, Vanham G. Essentials in saline pharmacology for nasal or respiratory hygiene in times of COVID-19. Eur J Clin Pharmacol. 2021; 77(9): 1275-1293. doi: 10.1007/s00228-021-03102-3. Epub 2021 Mar 27. Erratum in: Eur J Clin Pharmacol. 2021 Apr 24; PMID: 33772626; PMCID: PMC7998085.
- Kanjanawasee D, Seresirikachorn K, Chitsuthipakorn W, Snidvongs K. Hypertonic Saline Versus Isotonic Saline Nasal Irrigation: Systematic Review and Meta-analysis. Am J Rhinol Allergy. 2018; 32(4): 269-279. doi: 10.1177/1945892418773566. Epub 2018 May 18. PMID: 29774747.
- Ramalingam S, Cai B, Wong J, Twomey M, Chen R, Fu RM, et al. Antiviral innate immune response in non-myeloid cells is augmented by chloride ions via an increase in intracellular hypochlorous acid levels. Sci Rep. 2018; 8(1): 13630. doi: 10.1038/s41598-018-31936-y. PMID: 30206371; PMCID: PMC6134045.
- Machado RRG, Glaser T, Araujo DB, Petiz LL, Oliveira DBL, Durigon GS, et al. Hypertonic saline solution inhibits SARS-CoV-2 in vitro assay. bioRxiv. 2020.08.04.235549; doi: https://doi.org/10.1101/2020.08.04.235549
- Tatzber F, Resch U, Lindschinger M, Cvirn G, Wonisch W. Improved protection of filtering facepiece through inactivation of pathogens by hypertonic salt solutions - A possible COVID-19 prevention device. Prev Med Rep. 2020; 20: 101270. doi: 10.1016/j.pmedr.2020.101270. Epub 2020 Nov 28. PMID: 33282639; PMCID: PMC7695550.
- Ramalingam S, Graham C, Dove J, Morrice L, Sheikh A. A pilot, open labelled, randomized controlled trial of hypertonic saline nasal irrigation and gargling for the common cold. Sci Rep. 2019; 9(1): 1015. doi: 10.1038/s41598-018-37703-3. PMID: 30705369; PMCID: PMC6355924.
- Ramalingam S, Graham C, Dove J, Morrice L, Sheikh A. Hypertonic saline nasal irrigation and gargling should be considered as a treatment option for COVID-19. J Glob Health. 2020; 10(1): 010332. doi: 10.7189/jogh.10.010332. PMID: 32395245; PMCID: PMC7193539.
- Chatterjee U, Chakraborty A, Naskar S, Saha B, Bandyapadhyay B, Shee S. Efficacy of normal saline nasal spray and gargle on SARS-CoV-2 for prevention of COVID-19 pneumonia. Research Square Preprint. doi: 21203/rs.3.rs-153598/v1.
- Rosati P, Giordano U, Concato C. Hypertonic saline nasal irrigation and gargling as an inexpensive practical adjunctive weapon to combat asymptomatic SARS-CoV-2 infections. A case reports. Trends Medic. 2020; 20(6): 1-3. doi: 10.15761/TiM.1000249.
- Kimura KS, Freeman MH, Wessinger BC, Gupta V, Sheng Q, Huang LC, et al. Interim analysis of an open-label randomized controlled trial evaluating nasal irrigations in non-hospitalized patients with coronavirus disease 2019. Int Forum Allergy Rhinol. 2020; 10(12): 1325-1328. doi: 10.1002/alr.22703. Epub 2020 Oct 20. PMID: 32914928; PMCID: PMC7722064.
- Yildiz E, Koca Yildiz S, Kuzu S, Günebakan Ç, Bucak A, Kahveci OK. Comparison of the Healing Effect of Nasal Saline Irrigation with Triamcinolone Acetonide Versus Nasal Saline Irrigation alone in COVID-19 Related Olfactory Dysfunction: A Randomized Controlled Study. Indian J Otolaryngol Head Neck Surg. 2021: 1-6. doi: 10.1007/s12070-021-02749-9. Epub ahead of print. PMID: 34277384; PMCID: PMC8272442.
- Varricchio A, La Mantia I, Brunese FP, Varricchio A, Ciprandi G. Viral shedding in symptomatic patients with mild COVID-19: an experience with nebulized nasal treatment. J Biol Regul Homeost Agents. 2021; 35(3): 1155-1157. doi: 10.23812/21-137-L. PMID: 34233453.
- Ciprandi G, La Mantia I, Brunese FP, Varricchio A, Varricchio A. Hypertonic saline with xylitol and hyaluronate may shorten the viral shedding duration in asymptomatic COVID-19 positive subjects: a pilot study. J Biol Regul Homeost Agents. 2021; 35(3): 1151-1154. doi: 10.23812/21-138-L. PMID: 34229425.
- Varricchio A, La Mantia I, Brunese FP, Ciprandi G. Smell recovery in patients with COVID-19: an experience with nebulized nasal treatment. J Biol Regul Homeost Agents. 2021; 35(2): 683-686. doi: 10.23812/21-28-L. PMID: 33728831.
- Singh S, Sharma N, Singh U, Singh T, Mangal DK, Singh V. Nasopharyngeal wash in preventing and treating upper respiratory tract infections: Could it prevent COVID-19? Lung India. 2020; 37(3): 246-251. doi: 10.4103/lungindia.lungindia_241_20. PMID: 32367847; PMCID: PMC7353928.
- Shetty R, Lalgudi VG, Khamar P, Gupta K, Sethu S, Nair A, et al. Potential ocular and systemic COVID-19 prophylaxis approaches for healthcare professionals. Indian J Ophthalmol. 2020; 68(7): 1349-1356. doi: 10.4103/ijo.IJO_1589_20. PMID: 32587162; PMCID: PMC7574070.
- Panta P, Chatti K, Andhavarapu A. Does saline water gargling and nasal irrigation confer protection against COVID-19? Explore (NY). 2021; 17(2): 127-129. doi: 10.1016/j.explore.2020.09.010. Epub 2020 Oct 1. PMID: 33046408; PMCID: PMC7528968.
- Farrell NF, Klatt-Cromwell C, Schneider JS. Benefits and Safety of Nasal Saline Irrigations in a Pandemic-Washing COVID-19 Away. JAMA Otolaryngol Head Neck Surg. 2020; 146(9): 787-788. doi: 10.1001/jamaoto.2020.1622. PMID: 32722777.
- Stathis C, Victoria N, Loomis K, Nguyen SA, Eggers M, Septimus E, et al. Review of the use of nasal and oral antiseptics during a global pandemic. Future Microbiol. 2021; 16(2): 119-130. doi: 10.2217/fmb-2020-0286. Epub 2021 Jan 19. PMID: 33464122; PMCID: PMC7842245.
- Kramer A, Eggers M. Prävention respiratorischer Virusinfektionen durch viruzide Schleimhautantiseptik bei medizinischem Personal und in der Bevölkerung. Hygien Medizin Vol45 9/2020, D118-126.
- Leboulanger N, Sagardoy T, Akkari M, Ayari-Khalfallah S, Celerier C, Fayoux P, et al; French Association of Pediatric Otorhinolaryngology (AFOP); French Society of Otorhinolaryngology; Head, Neck Surgery (SFORL). COVID-19 and ENT Pediatric otolaryngology during the COVID-19 pandemic. Guidelines of the French Association of Pediatric Otorhinolaryngology (AFOP) and French Society of Otorhinolaryngology (SFORL). Eur Ann Otorhinolaryngol Head Neck Dis. 2020; 137(3): 177-181. doi: 10.1016/j.anorl.2020.04.010. Epub 2020 Apr 18. PMID: 32312676; PMCID: PMC7165275.
- Radulesco T, Verillaud B, Béquignon E, Papon JF, Jankowski R, Le Taillandier De Gabory L, et al. French Association of Rhinology (AFR); French Society of Otorhinolaryngology, Head and Neck Surgery (SFORL). COVID-19 and rhinology, from the consultation room to the operating theatre. Eur Ann Otorhinolaryngol Head Neck Dis. 2020; 137(4): 309-314. doi: 10.1016/j.anorl.2020.04.013. Epub 2020 Apr 30. PMID: 32387072; PMCID: PMC7190480.
- Kara E, Demİrkan K, Ünal S. Knowledge and Attitudes Among Hospital Pharmacists About COVID-19. Turk J Pharm Sci. 2020; 17(3): 242-248. doi: 10.4274/tjps.galenos.2020.72325. Epub 2020 Jun 22. PMID: 32636699; PMCID: PMC7336039.

