Assessment of the Monoclonal Antibody Palivizumab: Effects on Admission Rates, Cost-Effectiveness, and Severity for Premature Infants Attending RSV Clinic in King Hamad University Hospital, Kingdom of Bahrain
Dr. Mohamed Hegaze1, Dr. Ali Haider Ali2,*, Dr. Fatema Alahmed2, Dr. Sara Abduljawad2, Dr. Mariam Hamad Showaiter2, Dr. Yasmeen Abdulrahman Fayed2, Dr. Nasreen Sulaiman Omar Abdulaziz2 and prof. Gabriel Fox3
1Registrar of Pediatrics, King Hamad University Hospital, Kingdom of Bahrain
2Intern, Salamniya Medical Complex, Kingdom of Bahrain
3Consultant Pediatric Intensive Care, King Hamad Univeristy Hospital, Kingdom of Bahrain
Received Date: 07/08/2024; Published Date: 18/10/2024
*Corresponding author: Dr. Ali Haider Ali, Intern, Salamniya Medical Complex, Kingdom of Bahrain
Abstract
Background: Respiratory syncytial virus (RSV) is a leading cause of acute viral bronchiolitis in infants, particularly in pre-term infants who are at higher risk due to underdeveloped lungs and immune systems. Palivizumab, a monoclonal antibody, is recommended for preventing RSV infections in high-risk infants. This study evaluates the impact of palivizumab on admission rates, cost-effectiveness, and disease severity among premature infants attending the RSV clinic at King Hamad University Hospital in the Kingdom of Bahrain.
Methods: A retrospective analysis was conducted on 217 babies born at King Hamad University Hospital. Of these, 134 babies received Palivizumab in RSV clinic due to their gestational age or other risk factors that increased their likelihood of developing acute bronchiolitis, such as neuromuscular disease or airway disease (e.g., bronchopulmonary dysplasia). Additionally, 83 healthy babies, either term or preterm, who did not receive Palivizumab were included in the study. The data was collected from 2018 to 2022.
Results: A total of 134 (61.8%) received palivizumab. The majority of preterm infants (96.3%) received palivizumab, while only 8.4% of term infants did (p < 0.01). Receiving palivizumab was significantly associated with having a neuromuscular disease (6.0%, p = 0.025), airway Disease (10.4%, p < 0.01), and ER visits due to RSV (5.2%, p = 0.046). Pre-term infants had higher rates of airway disease (9.6% vs. 1.2%, p = 0.016) and emergency room visits due to RSV (5.1% vs. 0.0%, p = 0.047) compared to term infants. Only four pre-term patients were admitted to the pediatric ward, with no admissions to the PICU or complications from palivizumab.
Conclusion: While palivizumab appears to be appropriately targeted, its impact on reducing hospital admissions requires further investigation. The potential cost savings from reduced hospitalizations and severe RSV cases support further economic analysis.
Keywords: RSV; Bronchiolitis; Vaccine; Palivizumab; Airway Disease; Infectious Disease
Introduction
Infections are a common pediatric complaint worldwide, with most cases involving the upper and lower respiratory systems. Bronchiolitis is considered a common lower respiratory tract infection among children aged between 6 months and 2 years. Bronchiolitis is the inflammation and obstruction of the bronchioles, the smallest aspect within the respiratory tract [1,2]. Although it has multiple etiologies, it is mainly caused by a viral infection, almost exclusively by Respiratory Syncytial Virus (RSV), which is a major cause of morbidity and mortality among infants [2]. RSV is noted to be the cause of more than 3.3 million cases worldwide [3,4]. It is important to note that RSV is highly contagious, with viral shedding seen even after three weeks of infection [5]. Therefore, proper precautions must be taken to avoid the spread between infants in schools, communities, and neighborhoods.
Almost 2% of infants presenting to the emergency department are admitted to the hospital due to bronchiolitis, mainly in epidemic seasons such as winter [3]. Infants may present with a range of symptoms, including shortness of breath, wheezing, cough, or signs of respiratory distress, which may lead to the need for acute intervention such as mechanical ventilation secondary to respiratory failure [2]. Bronchiolitis is considered a clinical diagnosis, with a respiratory panel through a nasopharyngeal swab used when in doubt [5]. Management is usually supportive. Physicians tend to use pharmacological therapy such as nebulization therapy, steroids, and antibiotics [5].
Prophylactic therapy and use are of the utmost importance, especially for high-risk patients. These patients include infants with neuromuscular illness, preterm neonates, infants with congenital airway anomalies, and more [5-7]. Prophylactic therapy for any illness has a huge impact on decreasing morbidity, mortality, and overall chronic complications [5]. In terms of bronchiolitis, although no vaccine is available, the production of Palivizumab has created a shift in the prevention of bronchiolitis and its complications in general [5,6]. Approved by the Food and Drug Administration (FDA) in 1998, it is considered the main prophylactic agent for bronchiolitis caused by RSV in high-risk patients [6]. Palivizumab is a monoclonal antibody, usually administered as an intramuscular injection at a rate of once per month for five consecutive months within the age of 24 months [7]. The use of Palivizumab has been proven effective in decreasing admission rates within tertiary hospitals. In a study conducted in 2021, it was noted that the use of the monoclonal antibody reduced the rate of admissions in the pediatric population from 98 to 43 per 1000 [8]. Furthermore, a meta-analysis conducted in 2023 showed that Palivizumab has not only reduced admission rates but also decreased the use of supplemental oxygen and the admission rates to the pediatric intensive care unit [9]. To understand the cost-effectiveness of Palivizumab use, a study conducted in 2012 showed that its use has reduced costs on hospitals, leading to a more cost-effective approach to bronchiolitis management [10]. However, the cost-effectiveness of Palivizumab has been debated [11]. Therefore, this study aims to review the admission rates and severity of bronchiolitis among high-risk patients who received Palivizumab at King Hamad University Hospital between the years 2018 to 2022, to understand its impact on cost-effectiveness on the hospital.
Methods
Study Design and Population: A group of 217 babies were born at King Hamad University Hospital between 2018 and 2022. This group included 137 term and preterm infants (any neonate born before 37 weeks of gestation) who received Palivizumab. Data on any complications, such as the presence of neuromuscular illness, congenital airway anomalies, visits to the emergency department for reactive airway disease (any disorder affecting the airway tract due to overreaction to irritants leading to wheezing and shortness of breath), admissions to the general ward or pediatric intensive care unit, and total days of admissions, were collected through the information system at King Hamad University Hospital.
Statistical Analysis: Categorical variables were represented as frequencies and percentages. Associations between categorical variables were assessed using Chi-square and Fisher's exact tests. Analyses were conducted using SPSS (version 26.0) software. A p-value of less than 0.05 was considered statistically significant.
Ethical Consideration: Approval from the Ethical Committee within King Hamad University Hospital was received with the Institutional Research Board (IRD) RMS-BDF /P&PEC/ 2023-722. All precautions were taken to ensure the privacy of information of the participants and the data collected.
Results
Patient Characteristics: The analyses included a total of 217 babies born in king Hamad university hospital from 2018 – 2022; of those, 81 (37.3%) were term patient and 136 (62.7%) were pre-term. A total of 134 (61.8%) received the palivizumab. Frequencies and percentages of patients’ characteristics are represented in Table 1.
Table 1: Patients' Characteristics (N = 217).
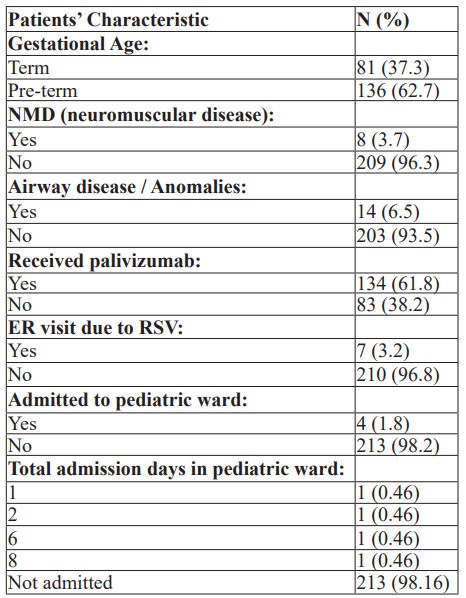
No patients were admitted to the PICU or had complications due to palivizumab.
Association with Receiving Palivizumab: Table 2 illustrates the association between patients' characteristics and receiving palivizumab.
Table 2: Association with Receiving Palivizumab.
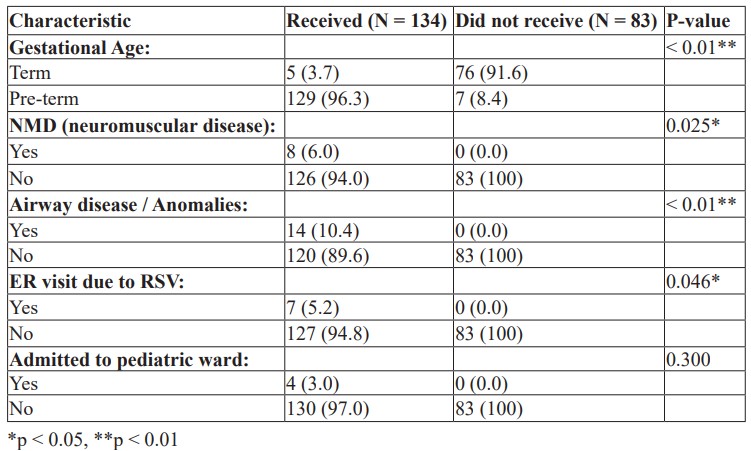
Patients’ gestational age had a statistically significant association with receiving palivizumab, with 96.3% of those who received palivizumab being pre-term, while only 8.4% of those who did not receive palivizumab being pre-term, and 91.6% of those who did not receive palivizumab were term patients. Receiving palivizumab had a statistically significant association with having NMD (6%), Airway diseases (10.4%), and visiting the ER due to RSV (5.2%), whereas none of those who did not receive palivizumab had these conditions or an ER visit, as illustrated in Table 2.
Association with Gestational Age: Table 3 shows the association between patients' characteristics and gestational age.
Table 3: Association with Gestational Age.
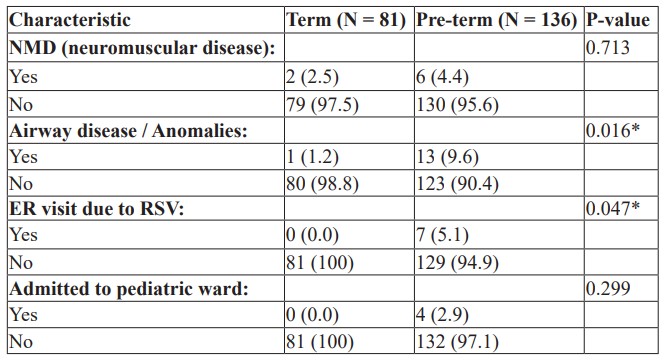
Patients’ gestational age had a statistically significant association with suffering from airway disease and ER visits due to RSV, with 9.6% of pre-term patients experiencing airway disease, while only 1.2% were term patients. 5.1% of pre-term patients had an ER visit due to RSV, whereas none of the term patients required an ER visit, as illustrated in Table 3. Only 4 patients were admitted to the pediatric ward and all of them were pre-term patients.
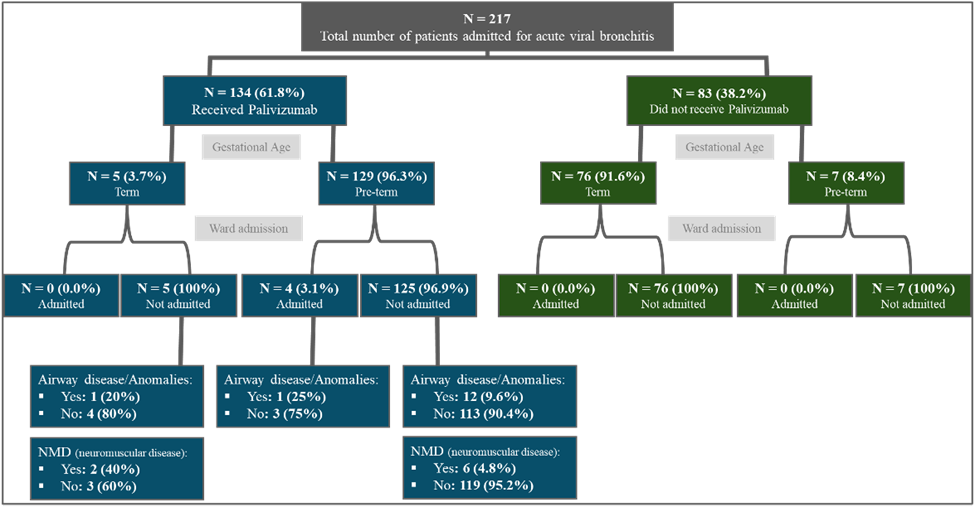
Figure 1: Shows the Summary of the Findings.
Discussion
The results indicate that pre-term infants are significantly more likely to receive palivizumab compared to term infants. This is consistent with the recommendations for palivizumab, which targets high-risk groups, including pre-term infants. The data also shows that patients with neuromuscular diseases and airway anomalies are more likely to receive palivizumab, highlighting the targeted use of this prophylactic treatment in high-risk populations. The study reveals that pre-term infants have a higher incidence of airway diseases and ER visits due to RSV compared to term infants. This underscores the increased vulnerability of pre-term infants to respiratory complications. However, the admission rate to the pediatric ward was low, with only four pre-term patients admitted, and no patients admitted to the PICU or experiencing complications due to palivizumab.
While this study does not include direct cost analysis, the significant reduction in severe RSV-related outcomes (e.g., ER visits and hospital admissions) among those receiving palivizumab suggests potential cost savings. By preventing severe infections and hospitalizations, palivizumab likely reduces the overall healthcare burden associated with RSV in preterm infants. The study is limited by its retrospective nature and the small sample size, particularly for certain outcomes like pediatric ward admissions. Future prospective studies with larger sample sizes are needed to validate these findings and assess the cost-effectiveness of palivizumab more comprehensively.
Conclusion
This study demonstrates that pre-term infants and those with specific comorbidities such as neuromuscular diseases and airway anomalies are more likely to receive palivizumab. These high-risk groups also show higher rates of airway diseases and ER visits due to RSV. The use of palivizumab appears appropriately targeted, though its impact on reducing hospital admissions needs further investigation. The potential cost savings from reduced hospitalizations and severe RSV cases warrant further economic analysis.
References
- Giersing BK, Karron RA, Vekemans J, Kaslow DC, Moorthy VS. Meeting report: WHO consultation on respiratory syncytial virus (RSV) Vaccine Development, Geneva, 25–26 April 2016. Vaccine, 2019; 37(50): 7355–7362.
- Silver AH, Nazif JM. Bronchiolitis. Pediatrics In Review, 2019; 40(11): 568–576.
- Smyth RL, Openshaw PJM. Bronchiolitis. The Lancet, 2006; 368(9532): 312–322.
- Bronchiolitis: An Update on Management and Prophylaxis – PubMed,
- Immunoprophylaxis of RSV Infection: Advancing from RSV-IGIV to Palivizumab and Motavizumab – PubMed, 2007.
- Monoclonal Antibody for the Prevention of Respiratory Syncytial Virus in Infants and Children: A Systematic Review and Network Meta-Analysis – PubMed, 2022.
- Palivizumab Injection: MedlinePlus Drug Information. (n.d.). MedlinePlus - Health Information from the National Library of Medicine.
- Viral Bronchiolitis – PMC. PubMed Central (PMC), 2019.
- Reeve CA, Whitehall JS, Buettner PG, Norton R, Reeve DM, Francis F. Cost‐effectiveness of respiratory syncytial virus prophylaxis with palivizumab. Journal of Paediatrics and Child Health, 2006; 42(5): 253-258.
- Weiner LB, Masaquel AS, Polak MJ, Mahadevia PJ. Cost-effectiveness analysis of palivizumab among pre-term infant populations covered by Medicaid in the United States. Journal of Medical Economics, 2012; 15(5): 997-1018.
- Mejías A, Ramilo O. Review of palivizumab in the prophylaxis of respiratory syncytial virus (RSV) in high-risk infants. Biologics: Targets and Therapy, 2008; 2(3): 433-439.

