Myxomatous Lesions in the Facial Bones: Case Series and Review of Literature
Kaoutar Youss1,*, Ouail Ilhami1,2, Bahaa Razem1, Abdelhakim Oukerroum1,2 and Faiçal Slimani1,2
1Service de Stomatologie et de Chirurgie Maxillo-faciale, Hôpital 20 Août, CHU Ibn Rochd, B.P, 2698, Casablanca, Morocco
2Faculté de Médecine et Pharmacie, Hassan II University of Casablanca, B.P, 5696, Casablanca, Morocco
Received Date: 20/05/2024; Published Date: 03/10/2024
*Corresponding author: Kaoutar Youss, Service de Stomatologie et de Chirurgie Maxillo-faciale, Hôpital 20 Août, CHU Ibn Rochd, B.P, 2698, Casablanca, Morocco
Abstract
Introduction: Myxomas are a rare entity of benign, locally aggressive tumors in the face and neck region, with a high rate of recurrence. The clinical, radiological and histological findings confirm the diagnosis. The treatment is mostly surgical.
Materials and Methods: This study of case series included 3 cases of facial myxomas in children. The characteristics were analyzed through a retrospective study with a detailed description of the clinical data, imaging, histology and treatment. A review of literature was also undertaken.
Results: One tumor was located in the maxilla, whereas 2 were located in the zygomatic bone. They were all treated by surgical resection. The diagnosis of myxoma was identified histopathologically. One patient presented a recurrence at 5 months of follow-up.
Conclusion: Although our series has a limited number of patients, it can be said that facial myxomas are specific in children. Our review of literature only confirms this data. Surgery is the treatment of choice and the choice of the approach depends mostly on the size and location of the tumor, as well as the preference of the surgeon.
Keywords: Myxoma; Jam myxoma; Children; Surgery
Introduction
Myxomas were first described by Virchow in 1863. They are benign, non-encapsulated, mesenchymal tumors that occur most frequently in the myocardium. Although they are slow-growing and non-metastasizing tumors, they are known to be locally very aggressive with a high rate of recurrence [1-3]. Should they arise in the head and neck region, the mandible is the most common localization followed by the maxilla, either deriving from the soft tissues or the facial bones [4]. The premolar and molar regions are the most commonly affected areas in comparison with the anterior region. They also involve the maxillary sinus when occurring in the maxilla, but most frequently the alveolar and zygomatic process [5,6]. Some authors reported rare cases of myxomas in the zygoma, skull base, palate, sphenoidal sinus, axis, temporal bone and nasal bone [7]. Other localization in soft tissues were reported such as the tongue, the larynx, the pharynx, the nose, the neck musculature, the cheek and the parotid gland [8,9]. They are recognized by the WHO under the entity of “odontogenic myxoma” although they can derive from mesenchyme and/or odontogenic ectomesenchyme with or without odontogenic epithelium [10,11].
Case 1
17-month-old child with no particular pathological history presented with a lateronasal swelling evolving for 2 months prior to admission, with no notion of pain or decline in general condition. Maxillofacial examination revealed a firm, painless, fixed right lateronasal swelling with a normal and mobile overlying skin, with an expansion to the upper-right vestibule, with no tooth mobility. The lymph nodes were free. The facial CT scan showed a lytic circumferential formation of the right maxilla, multilocular, mainly at the anterior wall of the maxillary sinus, extended to the frontal process of the maxilla and responsible for a bony swelling with thinning of the cortical bone and local rupture without invasion of the nasal cavities, orbit or jugal soft tissues. The patient had undergone enucleation of the maxillary mass via the superior vestibular approach. Macroscopically, the tumour was soft, friable and greyish. Microscopically, it revealed a proliferation of ovoid and spindle-shaped cells with no cyto-nuclear abnormalities or mytotic activity, arranged within a myxoid stroma and expressing neither desmin nor myogenin, in favor of a maxillary myxoma. Follow-up at 3, 6 and 12 months revealed no evidence of tumor recurrence.
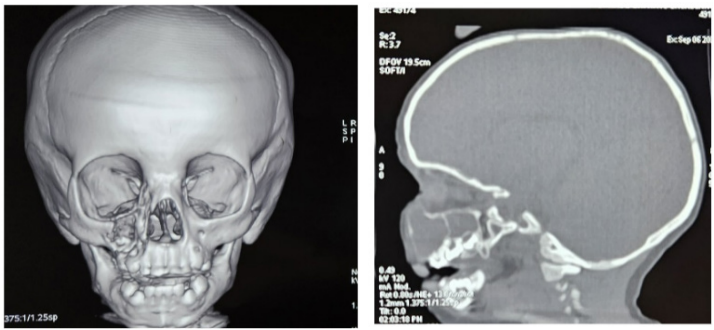
Figure 1: CT images showing the thinning bone by the lesion, and its limits.
Case 2
A 3-year-old child with no particular pathological history, presented with a swelling of the right cheek that had been evolving for 5 months prior to admission, with no pain or inflammatory signs. On admission, the child was in good general condition with good statural and weight development. Maxillofacial examination revealed a firm, painless, defined right suborbital and malar swelling, with a normal and mobile overlying skin, responsible for ectropion of the lower eyelid. Eye position and extraocular motion were preserved. The lymph nodes were free. A facial CT scan revealed an osteolytic lesion of the zygomatic bone, with extension to its zygomatico-temporal and zygomatico-frontal processes. The cortical bone was eroded, extending to the soft tissues and to the extraconical orbital fat, pushing the ocular globe medially without exophthalmos. The patient underwent a curettage of the lesion through a vestibular approach. The tumour was friable, greyish and easily dissectible from the underlying bone. Anatomopathological examination revealed a myxoid tumour proliferation with stellate cells expressing neither desmin nor myogenin, nor CD99 nor CD117, thus ruling out rhabdomyosarcoma and Ewing’s sarcoma. The patient was admitted 5 months later for a rapidly evolving tumor recurrence, this time compressing the eyeball and infiltrating the retro maxillo-zygomatic region. He underwent tumor resection using the previous approach.
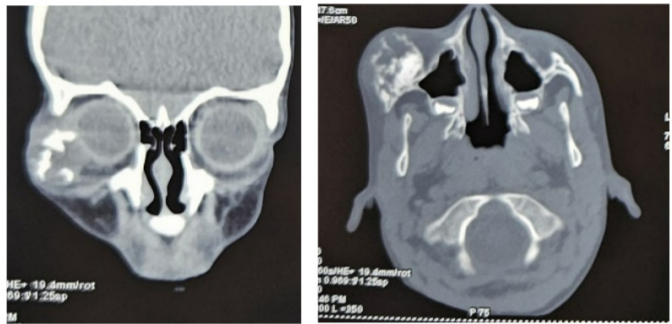
Figure 2: CT images showing an osteolytic lesion of the zygomatic bone with its extension in the orbit.
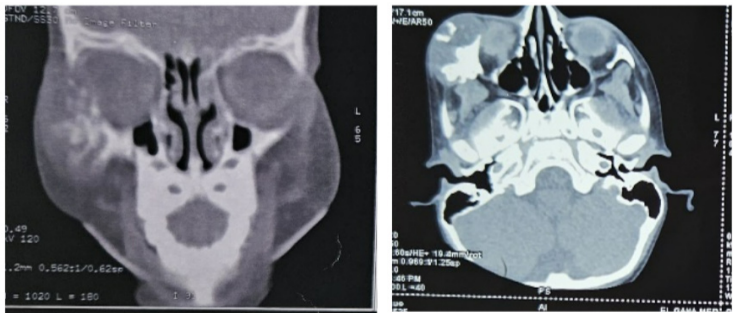
Figure 3: CT images showing the recurrent lesion and its extension in the orbit and the retro maxillo-zygomatic region.
Case 3
A 19-month-old child with no particular pathological history presented with a right malar swelling that had occurred 2 months prior to admission following a fall from his height, progressively increasing in volume with no associated pain or inflammatory signs. On admission, the child was in good general condition with good weight and height development. Maxillofacial examination revealed a firm, painless swelling of the right malar region, pushing the right ocular globe medially and causing narrowing of the palpebral fissure, without exophthalmos or oculomotricity disorders. The overlying skin was normal and mobile. The lymph nodes were free. A facial CT scan was performed, revealing an osteolytic lesion occupying the zygomatic bone, extending to its zygomaticotemporal process. It causes cortical breach and involves the extraconical fat, pushing the globe and the lateral rectus muscle inward without exophthalmos. The patient underwent a curettage of the mass via a medio-palpebral approach, which resulted in enucleation of a friable, beige-white tumour that was not adherent to the underlying bone. Anatomopathological examination revealed a fusocellular tumour proliferation on a myxoid background, with slightly atypical nuclei and no mitoses. On immunohistochemistry, the cells expressed AML without PS100, desmin, myogenin, CD34, CD31, beta-catenin or caldesmone. All these elements led to the diagnosis of myxoma. No signs of recurrence were detected during 12 months of follow-up.
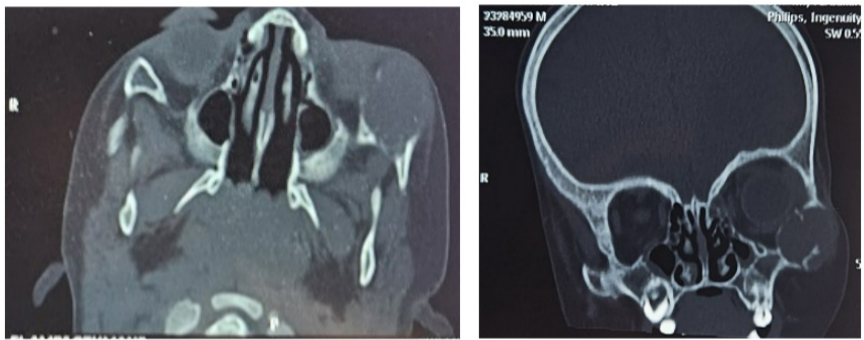
Figure 4: CT images showing the osteolytic lesion of the zygomatic bone extending to the orbit.
DIscussion
The neoplasm typically occurs between the second and fourth decade of life, rarely before the first and after the fifth decade [7,12-14]. Its occurrence rate is reported by Kaffe et al. to be as little as 7% before the age of 10 years old and even less in children younger than 18 months [15]. Although known for their slow growth, the case of infant myxomas (younger than 2 years of age) is particular since they tend to grow faster and have a slight preference for the maxilla [15-17]. Long asymptomatic, maxillary myxomas present as a hardened, non-tender, paranasal mass, associated with nasal congestion and/or obstruction, diplopia, dysesthesia, tooth mobility or chewing difficulty, depending on the extension of the mass [16,18,19].
Imaging lacks conclusiveness in this matter and is useful for the differential diagnosis as it allows the elimination of lesions that are dangerous to biopsy such as endonasal hemangioma, meningocele or meningoencephalocele, glioma [20,21]. Radiographically, Barros et al [22] suggested a two-stage progression of radiologic patterns. In the initial stage, an osteoporotic appearance is characterized by more pronounced medullary spaces separated by thin bone septa, appearing as “soap bubble”, “tennis racket” or “honeycomb” patterns. The subsequent stage involves a destructive phase marked by substantial expansion, loss of internal locules, and perforation of the cortical bone, leading to invasion into the adjacent soft tissues. The margins can present as well-defined, poorly-defined or diffuse based on the lesion borders [15,23].
On a macroscopic level, they manifest as a sizable white-grayish mass with indistinct boundaries, containing a gelatinous component internally, and exhibiting a firm consistency [4,14,24]. Histologically, the myxoma is characterized by the presence of ovoid, stellate, and spindle cells scattered in abundant myxoid stroma and a network of reticular fibers [24-27] . Collagen is typically found in limited quantities; however, in certain instances characterized as myxofibroma, higher levels of collagen may be observed. There is no atypia, and the mitotic activity is low [11, 27-30]. It has a poor vascularization [31]. The presence of odontogenic epithelium is not essential for the diagnosis. This appearance poses a problem for the differential diagnosis with myxoid liposarcoma, rhabdomyosarcoma, fibroblastic or chondroid tumors. The range of potential diagnoses also encompasses ameloblastoma, odontogenic fibroma, giant cell granuloma, fibrous dysplasia, and dentigerous cyst in the differential diagnosis [25,30,32]. Immunohistochemically, the tumor cells express vimentin but do not express cytokeratin, Neuron-Specific Enolase (NSE), Glial Fibrillary Acidic Protein (GFAP), neurofilaments, desmin [5,6,17,19,27,33].
Myxoma exhibits resistance to chemotherapy and displays only limited responsiveness to radiotherapy [18,34-36]. Thus, surgery remains the treatment of choice. Because of its 25% rate of recurrence, some authors agree on wide excision with safety margins of 10-15mm, while others recommend the use of adjuvant cryotherapy. In some cases, enucleation or simple curettage procedures can be carried out [5,16,25,27,37,38]. Brian et al. [5] reported zero recurrence in maxillary myxomas resected without margins, based on an average follow-up period of eight and a half years. For large tumors, extensive resection with maxillectomy can be performed [7,39,40]. The selection of the surgical approach is contingent on the tumor's location and extent. Maintaining close follow-up during the initial 2 years post-excision is crucial. Some even argue for a minimum follow-up duration of 5 years to confirm disease-free status [41,42].
Conclusion
Myxomas are benign and slow-growing tumors that are locally aggressive. On examination, they appear as a white-grayish mass, with a specific pattern on CT-Scan. Multiple diagnosis must be discussed before performing a biopsy. The diagnosis of myxoma is confirmed through the histological examination where stellate cells are found in a myxoid stroma. The treatment of choice is surgery but the recurrence rate is significantly high, therefore close follow-up post-surgery must be maintained.
Author contribution
Kaoutar Youss: Study concept, data collection, writing the paper and making the revision of the manuscript following the reviewer’s instructions.
Ouail Ilhami: Study concept and review
Bahaa Razem: study concept and review
Abdelhakim Oukerroum: Review
Faiçal Slimani: Reviewing and validating the manuscript’s credibility.
Competing interests: None
Funding statement: None
References
- Munjal N, Bharadwaj V, Garg B, Sood N. Odontogenic myxoma of the maxilla: A clinical case report and review of literature. Otolaryngol Online J, 2013; 3: 1-10.
- Carvalho de Melo AU, de Farias Martorelli SB, Cavalcanti PH, Gueiros LA, Martorelli Fde O. Maxillary odontogenic myxoma involving the maxillary sinus: Case report. Braz J Otorhinolaryngol, 2008; 74: 472-475.
- Lo Muzio L, Nocini P, Favia G, Procaccini M, Mignogna MD. Odontogenic myxoma of the jaws: a clinical, radiologic, immunohistochemical, and ultrastructural study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 1996; 82: 426e33.
- Odell EW, Adebiyi K. Odontogenic myxoma/myxofibroma. In: El-Naggar AK, Chan JKC, Grandis JR, et al., editors. World Health Organization Classification of head and neck tumors. 4th ed. Lyon: International Agency for Research on Cancer, 2017; pp. 229–30.
- Brian W. Rotenberg, Sam J Daniel, Iain A Nish, Bo Y Ngan, Vito Forte, Myxomatous lesions of the maxilla in children: a case series and review of management. International Journal of Pediatric Otorhinolaryngology, 2004; 68: 1251-1256.
- Keszler A, Dominguez FV, Giannuzio G. Myxoma in childhood: An analysis of 10 cases. J Oral Maxillofac Surg, 1995; 53: 518.
- Agbara Rowland, Fomete Benjamin, Obiadazie Athanasius-Chukwudi, Omeje Uchenna-Kevin, Samaila Modupeola-Omotara, Central Myxoma / Myxofibroma of the Jaws: A Clinico-Epidemiologic Review, Iranian Journal of Otorhinolaryngology, 2017; 29(1): 90.
- Faccini JM, Williams JL, Myxoma involving the soft tissues of the face, J. Laryngol. Otol, 1973; 87: 817 /822.
- Spengos MN, Schow CE, Myxomas of the soft tissues, J. Oral Maxillofac. Surg, 1965; 23: 140 /144.
- Truc Thi Hoang Nguyen, Mi Young Eo, Yun Ju Cho, Hoon Myoung, Soung Min Kim, Large myxomatous odontogenic tumor in the jaw: a case series. J Korean Assoc Oral Maxillofac Surg, 2021; 47: 112-119.
- Simon EN, Merkx MA, Vuhahula E, Ngassapa D, Stoelinga PJ. Odontogenic myxoma: a clinicopathological study of 33 cases. Int J Oral Maxillofac Surg, 2004; 33: 333-337. https://doi.org/10.1016/ j.ijom.2003.12.004.
- Wang K, Guo W, You M, Liu L, Tang B, Zheng G. Characteristic features of the odontogenic myxoma on cone beam computed tomography. Dentomaxillo-fac Radiol, 2017: 46: 20160232.
- Titinchi F, Hassan BA, Morkel JA, Nortje C. Odontogenic myxoma a clinico pathological study-in a South African population. J Oral Pathol Med, 2016; 45: 599-604.
- Etemad Moghadam S, Chookhachizadeh S, Baghaii F, Alaeddini M. Odontogenic myxoma: a study based on biopsy material over a 40-year period. J Contemp Dent Pract, 2014; 15: 137-141.
- Kaffe I, Naor H, Buchner A. Clinical and radiological features of odontogenic myxoma. Dentomaxillofac Radiol, 1997; 26: 299.
- Wachter BG, Steinberg MJ, Darrow DH, McGinn JD, Park AH. Odontogenic myxoma of the maxilla: a report of two pediatric cases. Int J Pediatr Otorhinolaryngol, 2003; 67: 389e-393.
- Rios Y Valles-Valles D, Vera-Torres AM, Rodriguez-Martinez HA, Rodriguez Reyes AA. Periocular myxoma in a child. Case Rep Ophthalmol Med, 2012.
- Parth Mewar, Karen E González‑Torres, Matthew Jacks T, Robert D Foss, Sinonasal Myxoma. A Distinct Lesion of Infants. Springer Head and Neck Pathology, 2020; 14: 212–219.
- Safadi A, Fliss DM, Issakov J, Kaplan I. Infantile sinonasal myxoma: a unique variant of maxillofacial myxoma. JOral Maxillofac Surg, 2011; 69(2): 553-558.
- Mukerji SS, Parmar HA, Gujar S. Intranasal meningoencephalocele presenting as a nasal polyp. A case report. Clin Imaging, 2011; 35: 309–311.
- Riffaud L, Ndikumana R, Azzis O, et al. Glial heterotopia of the face. J Pediatr Surg, 2008; 43: 1–3.
- Barros RE, Dominguez FV, Cabrini RL. Myxoma of the jaws. Oral Surg Oral Med Oral Pathol, 1969; 27: 225-236. https://doi. org/10.1016/0030-4220(69)90177-7.
- Noffke CE, Raubenheimer EJ, Chabikuli NJ, et al. Odontogenic myxoma: Review of the literature and report of 30 cases from South Africa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2007; 104: 101.
- Batsakis JG, Myxomas of soft tissues and the facial skeleton, Ann. Otol. Rhinol. Laryngol, 1987; 96: 618 /619.
- Kourda-Boujemaa J, Farah-Klibi F, et al. Odontogenic myxoma: About four cases and revue of literature. Ann Pathol, 2010; 30: 168–175.
- Sarode TP, Malik NA. Odontogenic myxoma in a child: Diagnostic and treatment dilemmas. J Indian Sot Pedod Prev Dent, 2002; 20: 68-72.
- Li TJ, Sun LS, Luo HY. Odontogenic myxoma: a clinicopathologic study of 25 cases. Arch Pathol Lab Med, 2006; 130: 1799-1806.
- Martínez-Mata G, Mosqueda-Taylor A, Carlos-Bregni R, de Almei da OP, Contreras-Vidaurre E, Vargas PA, et al. Odontogenic myxo ma: clinico-pathological, immunohistochemical and ultrastructural findings of a multicentric series. Oral Oncol, 2008; 44: 601-607. https:// doi.org/10.1016/j.oraloncology.2007.08.009
- Heffner DK. Problems in pediatric otorhinolaryngic pathology I. Sinonasal and nasopharyngeal tumours and masses with myxoid features. Int. 1. Pediat. Otorhinolaryngol, 1983; 5: 71-91.
- Dolores Rios y Valles-Valles, AM Vera-Torres, Case Report Periocular Myxoma in a Child. Hindawi Publishing Corporation Case Reports in Ophthalmological Medicine, 2012; 739094: 4 pages. doi:10.1155/2012/739094.
- Slootweg PB, vandenBos T, Strake W. Glycosaminoglycans in myxoma of the jaw: a biochemical study. J Oral Pathol, 1985; 14: 299-306.
- Regezi JA, Schibba J. Oral Pathology: Clinical Pathologic Cor relations, 3th ed. Philadelphia, PA: WB Saunders, 1999; 186e7
- Kadlub N, Mbou VB, Leboulanger N, et al. Infant odonto genic myxoma: a specific entity. J Craniomaxillofac Surg, 2014; 42: 2082–2086.
- Prasannan L, Warren L, Herzog CE, Lopez-Camarillo L, Frankel L, Goepfert H. “Sinonasal myxoma: a pediatric case,” Journal of Pediatric Hematology/Oncology, 2005; 27(2): pp. 90–92.
- James DR, Lucas VS, Maxillary myxoma in a child of 11 months, J. Craniomaxillofac. Surg, 1987; 15: 42-44.
- Ang HK, Ramani P, Miachels L. Myxoma of the maxillary antrum in children. Histopathology, 1993; 23: 361-365.
- King TJ, Lewis J, Orvidas L, et al. Pediatric maxillary odonto genic myxoma: A report of 2 cases and review of management. J Oral Maxillofac Surg, 2008; 66: 1057.
- Murphy C, Hayes R, McDermott GJ. Odontogenic myxoma of the maxilla: surgical management and case report. Ir J Med Sci, 2017; 186: 243-246.
- Brewis C, Roberts DN, Malone M, Leighton SEJ. Maxillary myxoma: a rare midfacial mass in a child. International Journal of Pediatric Otorhinolaryngology, 2000; 56: 207-209.
- Wankhedkar D, Patankar S, Gokul S, Sharma S. Odontogenic myxoma in an 8‑year‑old girl: A case report with review of literature. J Oral Maxillofac Pathol, 2019; 23: S83‑86.
- Wright JM, Soluk Tekkesin M. Odontogenic tumors: where are we in 2017? J Istanb Univ Fac Dent, 2017; 51(3 Suppl 1): S10-30. https://doi.org/10.17096/jiufd.52886
- Leiser Y, Abu-El-Naaj I, Peled M. Odontogenic myxoma–a case series and review of the surgical management. J Craniomaxillofac Surg, 2009; 37: 206–209.

