Intravesical Migration of An Intrauterine Device Complicated by Vesical Lithiasis: Triple Case Report
Seffar A*, Doumer A, Daghdagh Y, Kbirou A, Moataz A, Dakir M, Debbagh A and Abouateib R
Urology Department, CHU Ibn Rochd-Casablanca, Morocco
Received Date: 15/03/2024; Published Date: 05/08/2024
*Corresponding author: Seffar A, Urology Department, CHU Ibn Rochd-Casablanca, Morocco
Abstract
The Intrauterine Contraceptive Device (IUD) is the most widely used, safest and most reversible method of contraception because it is highly effective in regulating fertility, low-risk and does not require surgery. However, this contraceptive method is not free from complications such as pelvic discomfort, spontaneous expulsion, infection, and abnormal uterine bleeding. Perforation and migration are also part of the complications. These migrations may be undetected and misunderstood like fallen-out devices. A medical history suggesting the loss or disappearance of an intrauterine device in a patient with urinary symptoms should raise the suspicion of intravesical migration. Radiological examinations such as ultrasound and standard radiography of the urinary tract are useful methods for detecting IUD migration and encrustation of stones. Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) are other imaging modalities that can demonstrate the relationship of the IUD to adjacent structures and delineate fistulous pathways.
We present a report of 3 cases describing their characteristics and management of intrauterine device migration complicated by vesical lithiasis.
Keyword: Bladder stones in women; Intrauterine Device (IUD); Uterine perforation; Intra-vesical migration; Endourological management
Introduction
Intrauterine devices are a highly successful and patient-friendly method of long-term contraception when inserted and replaced or removed on time. The follow up after the procedure must be taken seriously. If this is not the case, several rare complications may arise. Uterine perforation is one of the most dangerous consequences of IUD use; nonetheless, it is uncommon and frequently asymptomatic, but it opens the door to more serious issues. Basically, this incidence typically occurs during insertion, however it may not be discovered until later if the patient attends her follow-up appointment, otherwise the IUD can migrate in many nearby organs. A literature review of the 18 years until 1999 found 165 documented cases distributed at the following sites: the omentum (27%), the rectosigmoid (26%), the peritoneum (24%), the appendix (0.05%), the small bowel (0.01%), the adnexa, and the iliac vein (0.006%), migration to the bladder is unusual, with only 31 cases reported [1].
Intravesical migration of an intrauterine device is a rare but potentially serious event that can occur in women with an intrauterine device (IUD). This is characterized by the displacement of the IUD from the uterine cavity into the bladder, leading to complications such as urinary symptoms, infections, and pelvic pain. Despite its rarity, it is important to understand the mechanisms and risk factors associated with this migration to effectively prevent and manage this complication. In this article, we present a report of 3 cases describing the management of intrauterine device migration forming an intra-vesical calculus.
Methods and Results
A case series examination of three cases of vesical migrating IUDs is presented. Their median age was 47 years. The instances were retrospective and nonconsecutive, and they were all presented in the same center. The data was collected from our hospital's registration database (2021-2023) following clearance from the study's ethical committee and informed written consent from the patients. Data privacy and security were considered throughout the project. This case series was reported in accordance with the PROCESS 2020 Guideline [2].
Case 1
49-year-old woman with 6 gravidity 4 parity (2 miscarriages). presented to our outpatient clinic with complaints of urinary urgency, frequency, and recurrent UTIs (Urinary tract infections). She reported that her urinary urgency and frequency had been present for the past 24 months. For the prior twenty-four months, she had been treated for multiple UTIs with Cefixime, ciprofloxacin and SMX-TMP for undocumented urinary tract infection. She had an intrauterine contraceptive device placed 20 years previously by a midwife one year after her third childbirth (she has given her fourth childbirth while she was using an IUD and the device was not found; it was wrongly concluded that it had probably been expulsed). The physical examination was unremarkable, the Internal visual exam under speculum did not reveal the IUD. Initial vital signs on presentation and laboratory test were all within normal limits. Urinalysis reveals a positive leukocyturia and hematuria, but the urine culture was negative. Abdominopelvic ultrasound revealed a 27 mm stone along the dome of the bladder. No IUD was found in the uterine cavity. KUB radiography allowed visualization of the IUD surrounded by the vesical stones, while the CT urogram described a bladder with multiple spontaneously hyperdense formations in the bladder dome generating artefacts, 1388UH measuring 21 x32.3x27.3mm associated with a sheet of effusion in the Douglas. The uterus was completely normal. The patient was managed in two stages:
- Initially, we performed cystoscopy with fragmentation of the stone using a YAG Holmium laser, combined with spectrophotometric infrared analysis of the fragments removed. We also performed four cold forceps biopsies of the bladder mucosa surrounding the IUD, which had an inflammatory appearance for fear of squamous cell carcinoma. Anatomopathological analysis revealed a morphological appearance of glandular cystitis (simple glandular metaplasia) with no sign of malignancy. Spectrophotometric analysis of the bladder stone fragments showed that the stone was composed of 97% calcium oxalate monohydrate (whewellite) and 3% calcium carbonate phosphate. The patient was discharged on Day 1 post-op and readmitted 14 days later for endoscopic removal of her IUD.
- Secondly, the intra-uterine contraceptive device that had migrated into the bladder was removed using a stone basket removal. There were no intra-operative complications, and the patient was discharged on day 2 post-op with a 16 CH bladder catheter with 5 days cefixime antibiotic.
At 7 days post-op, the patient was readmitted in emergency for urinary retention on the bladder catheter with signs of peritonitis. The urine dipstick came back positive for nitrates and leukocyte. The urine culture later found Escherichia coli. The CT urogram scan showed a moderately large peritoneal effusion. The patient was managed medically with triple antibiotic therapy consisting of ceftriaxon, gentamycin and metronidazole with good clinical and biological outcome and was declared discharged on day 5 post peritonitis. The bladder catheter was removed on day 21. After removal of the catheter, the woman experienced no pain, hematuria, or urinary burning. Two-month follow-up cystoscopy and KUB x-ray were normal (Figure 1).
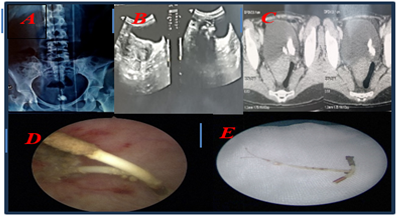
Figure 1: A: KUB x-ray showing calcific opacity and an IUD projecting into the pelvis; B: pelvic ultrasound: echogenic area with a posterior shadow cone and empty uterine cavity with no evidence of intrauterine device (IUD); C: CT scan showing the bladder stones, 1388UH measuring 21 x32.3x27.3mm; D: Endoscopic view showing IUD after laser fragmentation of the stones; E: The intrauterine device.
Case 2
A fifty-year-old four gravidity and four parities (all by vaginal delivery), presented to our outpatient consultation with complaints of six months hypogastric discomfort, pollakiuria, and burning urination but no fever. She claims to have had an IUD implanted in a health center by a midwife five years previously one year after her last delivery. Two years later, the IUD could not be discovered during a routine visit, and it was reported as a lost. She did not return for a follow-up; thus, no additional workup was undertaken. For the prior six months, she had been treated for multiple undocumented UTIs. The physical examination was unremarkable, the internal visual exam under speculum did not reveal the IUD.
An x-ray KUB was ordered with an ultrasound and an abdominal CT, which revealed an IUD surrounded by stones and bonded to the bladder wall. The stones were fragmented using laser YAG Holmium, followed directly by extraction of the IUD. The bladder catheter was left in place for three weeks. After removing the catheter, the woman had no pain, hematuria or burning of the urine and the control of KUB radiography was normal. Cystoscopy at two months was also normal (Figure 2).
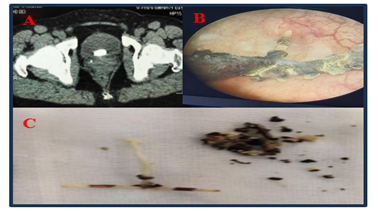
Figure 2: A: CT scan showing the encrusted intrauterine contraceptive device in the bladder; B: Endoscopic view showing calcified IUD; C: Extraction of IUD and fragments of stones.
Case 3
A 42-year-old three gravidity and three parities (all by vaginal delivery), presented to our outpatient clinic with complaints of ten months dysuria and pollakiuria, as well as intermittent terminal hematuria and she had been treated for multiple undocumented UTIs that had been developing for 10 months. She had an intrauterine contraceptive device implanted fourteen years prior to her presentation. four years later, the IUD could not be discovered during a routine visit, and it was reported as a lost IUD. She did not return for a follow-up; thus no additional workup was undertaken. The physical examination found an anterior vaginal prolapse grade 2 (cystocele) and the internal visual exam under speculum did not reveal the IUD. Abdominal ultrasound and KUB x-ray found out a 13 mm stone along the bladder dome, the abdominal CT scan revealed a blader stone of 17*16mm, 1290 HU, as well as a spontaneously hyperdense linear material measuring 38*5mm that encountered the vesical wall. An indication of laser fragmentation was given. The fragmentation of the stone with a Holmium YAG laser fiber was done. An endoscopic gripper was used to remove the broken piece of the IUD. The post-operative follow-up was simple. The KUB radiography show the persistence of extravesical spontaneously hyperdense linear material. The patient was subsequently referred to the gynecological department where the gynecology team made it possible to extract the rest of the intra uterine device by hysteroscopy. unfortunately, the patient was lost to follow-up (Figure 3).
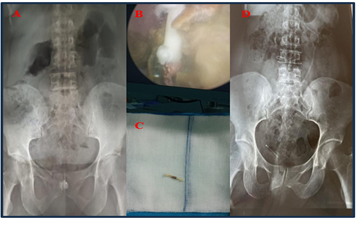
Figure 3: A: Pre-operative KUB x-ray showing calcific opacity projecting on the symphysis pubis and an IUD projecting into the right pelvis; B: Endoscopic view showing calcified IUD; C: Post-operative KUB x-ray showing the IUD projecting into the right pelvis.
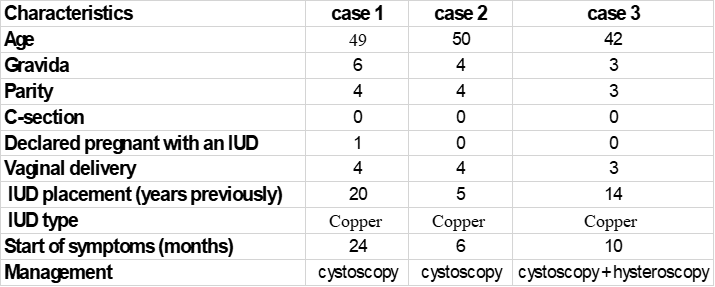
Table 1: Characteristics of the studied three cases.
Discussion
Among the 820 million married women of reproductive age who used any contraception in 2020, nearly half used permanent and long-acting methods (48 per cent), including female sterilisation (25 per cent) and IUDs (19 per cent). The total number of current IUD users has reached an estimated 161 million women worldwide [3].
The IUD might be considered the contraceptive method of choice for married women. There are two types of IUD: a copper IUD and a hormonal levonorgestrel device. Typical complications of both types include unsuccessful insertion, discomfort, vasovagal responses, infection, period irregularities, and expulsion. Although uncommon, uterine embedment (where the IUD is situated in the myometrium) and perforation (where any or the entire IUD is located beyond the uterine serosa) occur in approximately one of each 1000 insertions [4].
Known risk factors for uterine perforation include inadequate family planning provider training, insertion during the early puerperal phase when the uterus is fragile and bulky, a history of perforation, and an anatomically highly flexed uterus [5].
An IUD migrates because of a traumatic initial perforation of the uterus or a long-term inflammatory disease whose exact cause is unknown. Copper in some IUDs can cause an inflammatory response that results in the contraceptive effect, but it can also be involved in the process of long-term uterine perforation and transmigration [6]. It should be noted that our 3 patients wore copper-type IUDs.
But a large prospective non-interventional comparative cohort study of IUD users (N=61,448 women) revealed no significant difference in uterine perforation risk between hormonal and copper IUDs. Breastfeeding and proximity to a recent delivery (up to 36 weeks) were independently related with an increased risk of uterine perforation. The combination of these two factors was associated with an additive increase.
A migrating IUD's complications are mostly determined by where it ends up. A literature review of the 18 years until 1999 found 165 documented cases distributed at the following sites: the omentum (27%), the rectosigmoid (26%), the peritoneum (24%), the appendix (0.05%), the small bowel (0.01%), the adnexa, and the iliac vein (0.006%), migration to the bladder is unusual, with only 31 cases reported even though the bladder is very close to the uterus [1]. Reports of migrating IUDs causing ureteric erosion [7]and rectal perforation [8] have also been documented.
The interval between insertion and symptoms varies from 6 months to 16 years [9].
Bjornerem shows that IUDs can migrate to the bladder in a short amount of time. He described a case in which the patient had trouble of IUD. Lower abdominal pain and pollakiuria appears one week after IUD implantation. Three weeks later, cystoscopy confirmed that the IUD had been fully transported to the bladder, with intact bladder mucosa and no evidence of perforation. After migrating through the bladder wall, it frequently causes bladder irritation symptoms, and stones accumulate slowly over time. Its most common symptoms are frequent urination, urgency, dysuria, haematuria, and lower abdomen pain. [10]. These symptoms have been seen in each of our cases but very lately after the IUD placement.
It is unclear when the IUD migrated to the bladder: after insertion, intercourse, heavy work, or for unexplained reasons The transmigration of foreign bodies between organs is a highly intricate and challenging process to comprehend [11].
Regular follow-ups are therefore highly suggested for the early detection of IUD migration and the prevention of its consequences. The cause of this patients IUD migration was unknown, and they did not return for follow-up.
As recommended by the Moroccan ministry of health population department (family planning division), the first check should be performed four to six weeks after the insertion. Then, regular checks every 6 months aim to re-examine the patient outside of the rules to confirm the presence of IUD, ensure acceptance, and identify and treat secondary effects [12].
The IUD is usually removed in a consultation at the patient's request if she wishes to become pregnant, if the IUD is poorly tolerated or when the period of use has expired (Copper IUDs can last for as long as 10 years, while hormonal IUDs can be effective for three to eight years depending on the brand and type).
The majority of our patients did not comply with the follow-up, were not offered any change or removal of the IUD and mistakenly believed that the IUD had been expulsed. These three factors are what we believe to be the main risk factors associated with this complication.
The common rule is that any woman who complains of lower urinary tract issues with a notion that an IUD has been placed but not found or incorrectly presumed to have been expulsed is experiencing an intravesical migration of the IUD until the contrary is proved.
More than 50% of the published study including patients with IUD migration to the bladder have presented with stones ranging in size from 1 to 10 cm. It’s because the foreign bodies in the bladder cavity act as a nidus for stone formation, and infections may also be risk factors (urea-splitting organisms such as Proteus, Klebsiella, Serratia, and Enterobacter species produces alkaline urine, which promotes formation of struvite stones) [13]. It should be noted that all our patients were treated for undocumented recurrent urinary tract infections, which delayed the diagnosis.
Imaging plays a crucial role in the management of patients with migration IUDs. Ultrasonography is the most common initial method of evaluation due to its cost-effectiveness, lack of ionizing radiation, and greater detail of pelvic anatomy. The stem is usually easily identified on standard two-dimensional (2D) transvaginal ultrasonography (TVUS) as a linear echogenic structure. While the arms of the copper IUD are also fully echogenic, the arms of the levonorgestrel-releasing IUD are only echogenic at the proximal and distal ends, with characteristic central posterior acoustic shadowing on transverse images. Three-dimensional (3D) reconstructions are increasingly being used, particularly in the coronal view, which allows for a more careful evaluation of the arm positioning. When the IUD cannot be seen on pelvic ultrasonography, abdominal radiographs can be used to evaluate IUD positioning, as all IUDs are radiopaque. Positioning on an abdominal radiograph varies with normal uterine positions, but the IUD should be located near the midline low in the pelvis and orientated with the arms superior to the stem. In cases where complications such as perforations or abscesses are suspected, computed tomography (CT) or magnetic resonance imaging (MRI) may be a helpful adjunctive modality given their larger field of view. Of note, both copper and hormone-releasing devices are considered safe for up to 3-T MRI [14]. In all of our patients, the diagnosis was made during exploration.
Cystoscopic or suprapubic cystoscopic removal of the device and stones can be useful for IUDs that are fully inside the bladder or that develop small calculi [15]. Open surgery has usually been performed to remove IUDs that have formed large stones or with partial penetration of the bladder wall [16,17].
However, open surgery entails increased patient morbidity. Laparoscopy can be a less invasive option than an open surgical procedure [18].
Conclusion and Recommendation
Intrauterine devices can perforate the uterus and migrate into the bladder. Regular follow-ups are therefore highly suggested for the early detection this complication and prevention of its consequences.
It should be kept in mind that any woman who complains of recurrent lower urinary tract issues with a notion that an IUD has been placed or presumed expulsed without proof, is suffer from an intravesical migration of the IUD until the contrary is proved.
References
- Güvel S, Tekin MI, Kilinç F, Peskircioglu L, Ozkardeş H. Bladder stones around a migrated and missed intrauterine contraceptive device. Int J Urol, 2001; 8(2): 78-79. doi: 10.1046/j.1442-2042.2001.00249.x.
- Agha RA, Sohrabi C, Mathew G, Franchi T, Kerwan A, O’Neill N, et al. The PROCESS 2020 guideline: updating consensus preferred reporting of CasESeries in surgery (PROCESS) guidelines, Int. J. Surg, 2020; 84: 231–235.
- Goldstuck ND, Wildemeersch D. Role of uterine forces in intrauterine device embedment, perforation, and expulsion, Int. J. Women’s Health, 2014; 6: 735–744.
- Ezdi Sehar, Molitoris Joseph, Kantorova Vladimira. World Family Planning 2022 Meeting the changing needs for family planning: Contraceptive use by age and method, 2023.
- Ferguson CA, Costescu D, Jamieson MA, Jong L. Transmural migration and perforation of a levonorgestrel intrauterine system: a case report and review of the literature. Contraception, 2016; 83: 81–86.
- Akpinar F, Ozgur EN, Yilmaz S, Ustaoglu O. Sigmoid colon migration of an intrauterine device. Case Rep Obstet Gynecol, 2014; 2014: 207659.
- Kaneshiro B, Aeby T. Long-term safety, efficacy, and patient acceptability of the intrauterine Copper T-380A contraceptive device. Int J Women’s Health, 2010; 2: 211–220.
- Priyadarshi V, Sehgal N, Sen D. Ureteric erosion and obstruction: A rare but dreaded complication of intrauterine contraceptive device. Urol Ann, 2017; 9: 103–106.
- Abasiattai AM, Umoiyoho AJ, Utuk NM, Ugege W, Udoh IA. Intrauterine contraceptive device with rectal perforation and strings presenting at the anus. BMJ Case Reports, 2010; 2010: bcr0320102836. doi: 1136/bcr.03.2010.2836.
- Dietrick DD, Issa MM, Kabalin JN, Bassett JB. Intravesical migration of intrauterine device. J Urol, 1992; 147: 132–134.
- Bjornerem A, Tollan A. Intrauterine device–primary and secondary perforation of the urinary bladder. Acta Obstet Gynecol Scand, 1997; 76(4): 383–385. doi: 10.1111/j.1600-0412.1997.tb08000.x
- Rasekhjahromi A, Chitsazi Z, Khlili A, Babaarabi ZZ. Complications associated with intravesical migration of an intrauterine device. Obstet Gynecol Sci, 2020; 63(5): 675-678. doi: 10.5468/ogs.19105.
- Les standards des methodes de planification familiale au maroc edition 2007 royaume du maroc ministere de la sante direction de la population division de la planification familiale, 2007.
- Tosun M, Celik H, Yavuz E, Çetinkaya MB. Intravesical migration of an intrauterine device detected in a pregnant woman. Can Urol Assoc J, 2010; 4: E141-143.
- Nowitzki KM, Hoimes ML, Chen B, Zheng LZ, Kim YH. Ultrasonography of intrauterine devices. Ultrasonography, 2015; 34(3): 183-194. doi: 10.14366/usg.15010.
- Atakan H, Kaplan M, Erturk E. Intravesical migration of intrauterine device resulting in stone formation. Urology, 2002; 60: 911. doi: 10.1016/S0090-4295(02)01883-6.
- El-Hefnawy AS, El-Nahas AR, Osman Y, Bazeed MA. Urinary complications of migrated intrauterine contraceptive device. Int Urogynecol J Pelvic Floor Dysfunct, 2008; 19: 241–245. doi: 10.1007/s00192-007-0413-x.
- Maskey CP, Rahman M, Sigdar TK, Johnsen R. Vesical calculus around an intra-uterine contraceptive device. Br J Urol, 1997; 79: 654–655. doi: 10.1046/j.1464-410X.1997.00165.x.
- Shin DG, Kim TN, Lee W. Intrauterine device embedded into the bladder wall with stone formation: laparoscopic removal is a minimally invasive alternative to open surgery. Int Urogynecol J, 2012; 23(8): 1129-1131. doi: 10.1007/s00192-011-1632-8.

