Consecutive Cases of Ductal Adenocarcinoma of the Prostate Carrying HRR Mutation: Case Series and Literature Review
Tao Liu, Yongwen Luo, Yongzhi Wang, Weizhe Han, Nihati Rexiati and Zhonghua Yang*
Department of Urology, Zhongnan Hospital of Wuhan University, China
Received Date: 19/12/2023; Published Date: 06/05/2024
*Corresponding author: Zhonghua Yang, PhD, MD, Department of Urology, Zhongnan Hospital of Wuhan University, 169 Donghu Road, Wuhan 430071, China
Abstract
Background: Here we present the consecutive cases of ductal adenocarcinoma of the prostate carrying DDR mutations.
Cases presentation: In the 6 consecutive cases diagnosed recently in our center, 2 and 4 patients presented with PSA levels lower than 4 ng/ml and 10 ng/ml, respectively. Three of 6 were diagnosed by TURBT or TURP for presenting with a bladder lesion or a routine BPH. Only one case was diagnosed ductal adenocarcinoma of the prostate by biopsy and reaffirmed by pathology of radical prostatectomy. As for the symptoms, 3 of 6 patients presented with dysuria and frequency, while 2 of 6 complaining of gross hematuria. Among the 5 cases sequenced, 5 patients (100%) carried homologous recombination repair (HRR) related mutations, including BRCA1/2 in three patients, ATM, CHEK2 and CDK12 in one patient, respectively.
Conclusion: These are the first reports of 5 consecutive cases of ductal adenocarcinoma of prostate carrying HRR deficiency. The worse prognosis of ductal adenocarcinoma of the prostate may partly be attributed to the higher prevalence of HRR deficiency and personalized or precision therapies may play an important role in this rare type of prostate cancer.
Keywords: Prostate cancer; Ductal; Homologous recombination repair; DNA damage repair
Introduction
Ductal adenocarcinoma of the prostate (DAP) is the second most common histologic subtype of prostate cancer (PCa) [1]. It is more commonly mixed with acinar adenocarcinoma, being presented in 2.6% of prostatic adenocarcinomas [2]. Pure ductal type is even more rare, accounting for only 0.17% of prostatic adenocarcinomas [3].
Since DAP often presents at an advanced clinical stage with local or distant metastases and no effective treatment has been established, the prostate-specific mortality (PSM) is significantly worse than that of acinar adenocarcinomas of prostate (AAP) with conventional therapies, including radical prostatectomy (RP), radical radiotherapy (RT) and hormonal therapy (HT) [4].
Homologous recombination repair (HRR) is a tightly coordinated pathway that enables cells to control and regulate DNA damage that arises every day. Alterations in HRR genes are found to be frequently mutated in many types of cancer. About 23–31% of patients with advanced prostate cancer have been reported to have alterations in HRR genes [5]. Based on the principle of synthetic lethality, prostate cancer patients of advanced stage carrying deficiency in the HRR pathway responded to PARP inhibitors and several PARP inhibitors have demonstrated efficacy in patients with deficiency in the HRR pathway in the tumour and have been approved for treatment of HRRm mCRPC [6].
Here we present 6 consecutive cases of DAP recently diagnosed in our center, of which 5 patients were sequenced and all harbouring one or more HRR-related gene alterations. By means of next-generation sequencing (NGS), personalized or precision therapies may play an important role in this rare type of prostate cancer.
Case Series
Case 1: A 59-year-old man presented with dysuria with an HIV history and no cancer-related family history. His total and free serum prostate-specific antigen (PSA) were 3.899 ng/ml and 1.411ng/ml,respectively. Computed tomography urography (CTU) demonstrated a mass located at bladder neck, 10 × 9 × 10 mm in size, and magnetic resonance imaging (MRI) of the pelvis showed thicken urethral with nodules and significant enhancement throughout the entire process, suggesting the possibility of granulomatous lesions in the posterior urethra. Transurethral resection of bladder tumor (TURBT) detected DAP invading bladder and hence, radical cystectomy was performed. Pathological examination revealed mixed prostate adenocarcinoma (Gleason score 4+4) at a final staging of pT4N0M0, of which 80% of tumour was ductal adenocarcinoma and 20% of acinar adenocarcinoma. DNA sequencing identified CDK12 and BRCA1 mutations (Table 1). To prevent disease progressing, androgen deprivation therapy (ADT) combination with PARP inhibitor were administrated 6 weeks postoperatively, with an undetectable PSA level.
Table 1: Result of NGS of 5 Cases.
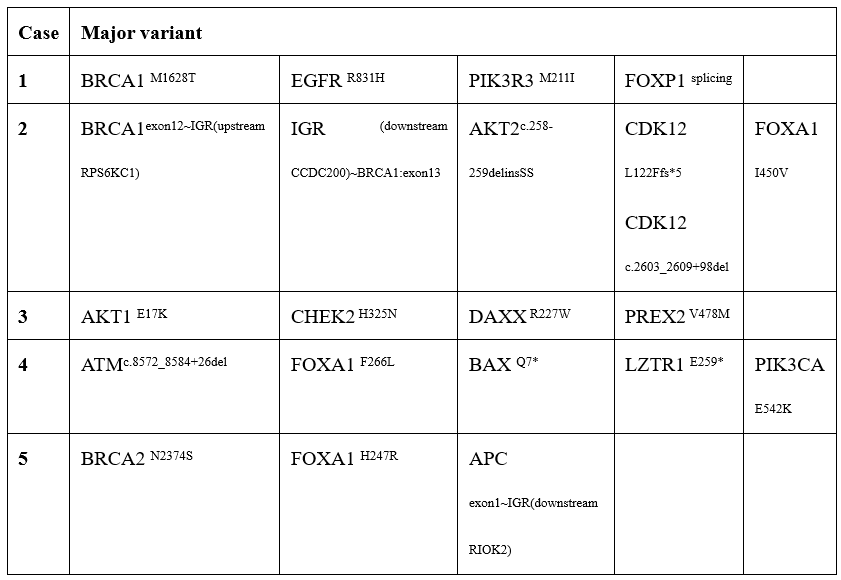
Case 2: A 75-year-old man presented with dysuria and frequency with a PSA value of 2.78 ng/ml. The patient received transurethral resection of prostate (TURP) for lower urinary tract symptoms (LUTS) and pathology examination revealed PDA (Gleason score 4+4). After excluding the regional or metastatic lesion, a radical prostatectomy was performed 4 weeks later. The final pathology result demonstrated features of mixed prostate carcinoma, of which, DAP accounting for over 85% of lesion resected and a staging of pT3bN0M0. DNA sequencing identified a mutation of CHEK2 (c.973C > A, p. H325N). This patient experienced persistent PSA (0.17ng/ml) and biochemical recurrence (BCR) 8 weeks and 5 month postoperatively, respectively. PSMA PET-CT detected several lesions in both lungs, then ADT combination with abiraterone acetate (AA), a novel Androgen receptor signaling inhibitor, were administrated because of low abundance of mutation in CHEK2.
Case 3: A male patient of 72-year-old presented with frequency and urgency with a hypertension history. The total PSA was 9.69 ng/ml with a f/t ratio of 0.13 then, and the total PSA had never been higher than 3.0 ng/ml before. MRI of the prostate demonstrated diffuse mixed signals and several node projecting into the bladder from the prostatic transitional zone. Pathology of prostate biopsy revealed prostate adenocarcinoma (Gleason score 4+4), however, the pathology was confirmed to be prostate ductal adenocarcinoma (Gleason score 4+5) after RP. The final pathological stage was pT4N1M0. Subsequent DNA sequencing identified ATM mutation (c8572_8584+25del). The patient received salvage radiotherapy combination with ADT 6 weeks postoperatively for a PSA value of 2.25 ng/ml.
Case 4: A 68-year-old man presented with gross hematuria. The PSA level of this patient is 11.068 ng/ml and CTU demonstrated a lesion in the bladder neck and trigone. Prostate biopsy and TURBT revealed prostate adenocarcinoma, and sequent radical cystectomy demonstrated mixed prostate adenocarcinoma, most of which was DAP (Gleason score 5+4). Six months later, two of his brothers were diagnosed with cancer, one for prostate cancer, the other for lung cancer. DNA sequencing identified a germline mutation of BRCA1(M1628T). The patient received no further treatment 4 weeks postoperative when PSA remained at 2.036 ng/ml and developed metastasis in both lungs one year later.
Case 5: A man of 62-year-old presented with frequency and dysuria and an elevated value of PSA (191ng/ml). Pelvic MRI demonstrated diffuse abnormal signals in prostate and seminal vesicles, and biopsy of prostate revealed prostate adenocarcinoma (Gleason Score 4+4). Routine radiography did not detect remote metastasis and radical prostatectomy was performed. Final pathologic examination demonstrated mixed prostate adenocarcinoma, of which ductal adenocarcinoma accounting for 90% of resected prostate. DNA sequencing identified a mutation of BRCA2(c.7121A>G, p.N2374S). As a pathological staging of pT3bN1M0 and a PSA level of 27.5 ng/ml 6 weeks postoperative, the patient was administrated ADT combination with abiraterone acetate.
Case 6: A man of 70-year-old presented with gross hematuria. The total PSA was 8.415 ng/ml and a f/t ratio of 0.73. CTU and pelvic MRI demonstrated lesions in the prostate and bladder neck. Although subsequent TURBT revealed mixed prostate adenocarcinoma (75% DAP) invading the muscle layer of the bladder, the simultaneous 13-core prostate biopsy could not detect any tumor.
Discussion
DAP is an uncommon variant of prostatic carcinoma with aggressive behavior and worse prognosis. Compared with AAP, DAP is reported to have a significantly lower PSA levels at diagnosis [7]. In our series of 6 consecutive patients, two and four patients presented with PSA levels less than 4 ng/ml and 10 ng/ml, respectively. On the other hand, DAP exhibits unique clinicopathological and radiological features, which are not clearly appreciable on preoperative imaging or biopsy [8]. As in our series, 3 of 6 were diagnosed by TURBT or TURP for presenting with a bladder lesion (Case 1, 6) or a routine BPH (Case 2). Two patients were diagnosed with prostate adenocarcinoma by prostate biopsy but confirmed as DAP by subsequent RP (Case 3 and 4). Only one case was diagnosed DAP by prostate biopsy and reaffirmed by pathology of RP (Case 5), which had a PSA value of 191 ng/ml.
As DAP often originates from primary periurethral prostate ducts, patients with this type of tumor often complain of urethral obstruction or hematuria at the time of disease presentation. As in our series, 3 of 6 patients presented with dysuria and frequency, while 2 of 6 complaining of gross hematuria. Specially in case 6, we detected DAP by TURBT but got no signal of tumor by prostate biopsy. This can be partly explained that as described above, DAP often originates from primary periurethral prostate ducts surrounding urethral where is detoured in the procedure of prostate biopsy.
Compared with conventional acinar PCa, DAP is usually associated with a worse prognosis. Patients undergoing conventional therapy, including radical prostatectomy (RP) or radical radiotherapy (RT) for DAP, had worse outcomes than AAP patients, whether receiving neoadjuvant or adjuvant therapy or not [9-10]. Upregulation of several intrinsic resistance pathways in DAP might render ADT less effective [10].
Deficiencies in HRR genes are actionable in approximately 25% of metastatic castration-resistant prostate cancers (mCRPC) patients, while they are relatively rare in metastatic hormone-sensitive prostate cancer (mHSPC) or local prostate cancer [11-13]. In our series, 5 of the 5 patients (100%), all at local or locally advanced stage, carried HRR-related mutations, including BRCAs in three patients, ATM, CHEK2 and CDK12 in one patient, respectively. We postulate that the worse progonsis of DAP may partly be attributed to the higher prevalence of HRR mutation.
Currently, there are no clear guidelines for the treatment of DAP and the treatment protocol for AAP with the same Gleason Score is used for DAP. Studies have shown that surgical treatment can achieve a longer survival time than radiotherapy or systemic chemotherapy, especially in patients with non-metastatic DAP [14].
Conclusion
To conclude, we presented the first series of 6 DAP cases, of which samples from 5 patients were sequenced and all (100%) carried HRR-related mutations, including BRCAs, ATM, CHEK2 and CDK12 even in local or locally advanced stage. We postulate that the worse prognosis of DAP may partly be attributed to the higher prevalence of HRR mutations.
Author Contributions
Tao Liu and Yongwen Luo: Data curation, Investigation, Methodology, Resources, Validation and Visualization. Yongzhi Wang and Weizhe Han: Data curation, Formal analysis, Investigation, Methodology and Resources. Nihati Rexiati: Validation, Visualization, Writing - review & editing. Zhonghua Yang: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft. All the authors have reviewed the paper and approved the final version.
Conflict of interests: None.
Acknowledgements: This study was supported by the research fund from medical Sci-Tech innovation platform of Zhongnan Hospital, Wuhan University (PTXM2020005).
References
- Knipper S, Preisser F, Mazzone E, Mistretta FA, Tian Z, Briganti A, et al. Contemporary Comparison of Clinicopathologic Characteristics and Survival Outcomes of Prostate Ductal Carcinoma and Acinar Adenocarcinoma: A Population-Based Study, 2019; pp. 231-237.e2.
- Seipel AH, Wiklund F, Wiklund NP, Egevad L. Histopathological features of ductal adenocarcinoma of the prostate in 1,051 radical prostatectomy specimens. Virchows Archiv : an international journal of pathology, 2013; 462(4): 36-429.
- Ranasinha N, Omer A, Philippou Y, Harriss E, Davies L, Chow K, et al. Ductal adenocarcinoma of the prostate: A systematic review and meta-analysis of incidence, presentation, prognosis, and management. BJUI compass, 2021; 2(1): 13-23.
- Ranasinghe W, Shapiro DD, Hwang H, Wang X, Reichard CA, Elsheshtawi M, et al. Ductal Prostate Cancers Demonstrate Poor Outcomes with Conventional Therapies, 2021; pp. 298-306.
- Robinson D, Van Allen EM, Wu Y, Schultz N, Lonigro RJ, Mosquera J, et al. Integrative clinical genomics of advanced prostate cancer, Cell, 2015; 161(5): 1215-1228.
- Herzog TJ, Vergote I, Gomella LG, Milenkova T, French T, Tonikian R, et al. Testing for homologous recombination repair or homologous recombination deficiency for poly (ADP-ribose) polymerase inhibitors: A current perspective. European journal of cancer (Oxford, England: 1990), 2023; 179: 136-146.
- Morgan TM, Welty CJ, Vakar-Lopez F, Lin DW, Wright JL. Ductal adenocarcinoma of the prostate: increased mortality risk and decreased serum prostate specific antigen. The Journal of urology, 2010; 184(6): 7-2303.
- Giganti F, Allen C, Sridhar A, Tandogdu Z, Ramachandran N, Dickinson L, et al. Mixed acinar and macrocystic ductal prostatic adenocarcinoma, 2021; p. e37.
- Tan YG, Khalid F, Huang HH, Chen K, Tay KJ, Lau WKO, et al. Prostatic ductal adenocarcinoma variant predicts worse pathological and oncological outcomes: Insight from over 1000 consecutive patients from a large prospective uro-oncology registry. The Prostate, 2021; 81(4): 242-251.
- Ranasinghe W, Shapiro DD, Hwang H, Wang X, Reichard CA, Elsheshtawi M, et al. Ductal Prostate Cancers Demonstrate Poor Outcomes with Conventional Therapies, 2021; pp. 298-306.
- Ghose A, Moschetta M, Pappas-Gogos G, Sheriff M, Boussios S. Genetic Aberrations of DNA Repair Pathways in Prostate Cancer: Translation to the Clinic. Int J Mol Sci, 2021; 22(18).
- Boussios S, Rassy E, Shah S, Ioannidou E, Sheriff M, Pavlidis N. Aberrations of DNA repair pathways in prostate cancer: a cornerstone of precision oncology, 2021; pp. 329-333.
- Burdak-Rothkamm S, Mansour WY, Rothkamm K. DNA Damage Repair Deficiency in Prostate Cancer. Trends Cancer, 2020; 6(11): 974-984.
- Bronkema C, Arora S, Keeley J, Rakic N, Sood A, Dalela D, et al. Impact of treatment modality on overall survival in localized ductal prostate adenocarcinoma: A national cancer database analysis. Urologic oncology, 2021; 39(6): 366.e11-366.e18.

