Late Presentation of Acute Pulmonary Embolism following Outpatient-treated Mild COVID-19
Avinash Singh1,*, Jeeyune Bahk2, Yesha Patel Rana3, Andrea Monfasani4, Jason Filopei5, Jigna Zatakia5, Lauren Blackwell5 and David Steiger1
1Division of Pulmonary, Critical Care & Sleep Medicine, Mount Sinai Morningside-West-Beth Israel Hospitals, Icahn School of Medicine at Mount Sinai, New York
2Division of Internal Medicine, Mount Sinai Morningside and Mount Sinai West Hospitals, Icahn School of Medicine at Mount Sinai, New York
3Division of Internal Medicine, Mount Sinai Beth Israel Hospital, Icahn School of Medicine at Mount Sinai, New York
4Division of Pulmonary, Critical Care & Sleep Medicine, Mount Beth Israel Hospital, Icahn School of Medicine at Mount Sinai, New York
5Division of Pulmonary, Critical Care & Sleep Medicine, Mount Sinai Beth Israel Hospital, Icahn School of Medicine at Mount Sinai, New York
Received Date: 08/08/2023; Published Date: 22/12/2023
*Corresponding author: Avinash Singh, Division of Pulmonary, Critical Care & Sleep Medicine, Mount Sinai Morningside-West-Beth Israel Hospitals, Icahn School of Medicine at Mount Sinai, New York, USA
Abstract
Background: COVID-19 is associated with coagulopathies and venous thromboembolism (VTE), particularly in patients hospitalized with a critical illness.
Methods: A retrospective, observational case series of adult patients admitted to three hospitals between 2/1/2020 and 4/1/2021, who were diagnosed with an acute pulmonary embolism (PE), following previous COVID-19. 24 patients were diagnosed with a symptomatic PE, of whom 18 (75%) had not required hospitalization for COVID-19 treatment.
Results: Median time from last positive COVID-19 test to diagnosis of PE was 151(± 122) days. Three patients (13%) were diagnosed with PE as outpatients. 52% and 38% were at low and intermediate risk of mortality, respectively. Five (24%) had a proximal deep venous thrombosis. All patients had an elevated D-dimer, elevated Factor VIII levels were detected in 46%, and 29% had positive lupus anticoagulant and elevated anti-cardiolipin antibody IgM levels. None required advanced therapy. There was no major bleeding and all patients survived to discharge. On outpatient follow-up, most patients were symptomatic, mostly with dyspnea (96%).
Conclusion: PE should be considered in patients diagnosed with COVID-19 many weeks earlier, including patients previously managed as outpatients, who have acute symptoms of dyspnea or chest pain, persistent elevated D-dimer elevation or Factor VIII, or clinical risk factors for VTE. Studies are required to evaluate the efficacy and safety of thromboprophylaxis in patients diagnosed with COVID-19 as outpatients.
Keywords: Coronavirus disease 2019 (COVID-19); Pulmonary embolism; Coagulopathy; Outpatient
Introduction
Coronavirus disease 2019 (COVID-19) is caused by a novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [1]. The disease severity ranges from an asymptomatic state to severe pneumonia, ARDS, multiorgan failure [2], and long-term complications (long COVID) [3]. Patients with severe COVID-19 develop coagulopathies associated with elevated D-dimer levels, venous thromboembolism (VTE), disseminated intravascular thrombosis (DIC), and bleeding [4]. Mechanisms include endothelial dysfunction, as evidenced by elevated von Willebrand factor (vWF) and Factor VIII levels, and prothrombotic state caused by proinflammatory cytokines [5-8]. High VTE rates of 21% to 69% in critically ill patients with COVID-19 have been described, with rates greater than 20-30% occurring despite prophylactic anticoagulation [9-11].
Many guidelines address thromboprophylaxis and risks and benefits of extended thromboprophylaxis, to minimize the risk of post-discharge VTE events [12-14]. Post-discharge thromboprophylaxis is suggested in patients with low bleeding risk [12] and additional risk factors for developing VTE including critical illness, diminished mobility/weakness, and prolonged sedation/paralysis [14]. In a recent study of symptomatic clinically stable outpatients with COVID-19, patients randomized to aspirin, low or therapeutic dose of apixaban therapy did not experience a decrease in the rate of venous or arterial thrombosis compared to placebo [15]. There is a paucity of literature describing VTE after hospital discharge in patients with COVID-19 [16-18] particularly in those treated for COVID-19 as outpatients.
We conducted a retrospective analysis of 24 consecutive patients admitted with acute VTE, following either inpatient or outpatient management of COVID-19. Our hypothesis was that a significant number of patients, who did not have a prior VTE or thrombophilia family history, are at an increased risk of developing an acute VTE weeks to months after the acute illness.
The aim of the study was to define possible predisposing factors to development of VTE following SARS-CoV-2 infection. We hoped to identify patients with acute COVID-19 treated either as inpatients or outpatients, who may benefit from extended thromboprophylaxis.
Methods
1. Study design, setting, and population
This retrospective, observational, case series included patients admitted to three Mount Sinai Health System Hospitals in New York City – Mount Sinai Morningside, West, and Beth Israel (including Mount Sinai National Jewish Respiratory Institute), between February 1, 2020 and April 1, 2021, who were diagnosed with VTE, after previously testing positive for SARS-CoV-2 on qualitative reverse-transcriptase polymerase chain reaction (RT-PCR) assay. Inpatient cases were identified from those who received a Pulmonary Medicine/Critical Care consult for acute VTE. Outpatient cases were identified from those who presented to the Pulmonary Medicine clinic for a follow-up of a recently diagnosed VTE. Of these, patients who had a VTE imaging available for review, and had a previous positive test result for SARS-CoV-2 available in their medical records, were included in this study.
A total of twenty-four adult patients met our inclusion criteria. Pregnant women, children (below 18 years of age), and patients with acute VTE with no documented positive SARS-CoV-2 PCR result in our medical records were not included. The institutional review board of Mount Sinai Health System approved this study. Informed consent was waived, and researchers exclusively utilized de-identified data.
2. Data collection
Clinical data was accessed via the electronic medical record system, EPIC. De-identified data were extracted from medical charts including demographics, medical history, symptoms, laboratory, imaging results, and length of stay (LOS). Data collected from outpatient records also included diagnostic tests for thrombophilia, presence and levels of circulating IgG anti- SARS-CoV-2 (COVID-19 ELISA IgG Antibody Test, developed at the Mount Sinai Laboratory). Coexisting medical conditions and presenting symptoms were obtained from physician documentation. All laboratory and imaging tests were performed at the discretion of the treating physician.
3. Statistical analysis
Time between COVID-19 illness and VTE diagnosis was calculated using the dates of first documented positive nasopharyngeal RT-PCR for SARS-CoV-2, and Chest CT Angiogram (CTA) that identified an acute Pulmonary Embolism (PE). Descriptive statistics was utilized to summarize the data. We report continuous variables as medians or means and categorical variables as counts or percentages. No adjustment was made for missing data. Analysis was performed with Stata 15.1*** software (StataCorp).
Results
1. Characteristics of COVID-19 – positive patients (demographics)
Median age of patients was 57 years; the majority (54%) were female. Most (62%) patients were obese based on Body Mass Index (BMI>/=30 Kg/m2) noted at admission. Most were Caucasian (33%), African-American (29%), or Hispanic (29%). Half of the patients had hypertension, followed by COPD/Asthma (25%), Diabetes mellitus (21%) and Obstructive Sleep Apnea (OSA) (21%). Two (8%) had active malignancy. Three (12.5%) patients were on long-term systemic anticoagulation for prior VTE, whereas four (17%) were on single antiplatelet therapy. Three (12.5%) endorsed active smoking at the time of COVID-19 diagnosis.
2. Characteristics and outcomes of hospitalized patients for COVID-19 (COVID-19 admission, and disease severity)
Among patients diagnosed with COVID-19, six (25%) required hospital admission. When tested for biochemical markers, all patients demonstrated elevated levels of D-dimer, C-reactive protein (CRP), and interleukin-6 (IL-6). None had elevated procalcitonin values. Thrombocytopenia (<150K/uL) and elevated creatinine from baseline were noted in half (50%) and a third (33.3%), respectively. None required ICU-level of care.
A third (33%) of inpatients required advanced oxygen support in the form of High Flow Nasal Oxygen (33%) or Bilevel Noninvasive Ventilation (17%). All received corticosteroids as a standard-of-care, with the addition of hydroxychloroquine (83%) and azithromycin (50%). One patient each received convalescent plasma and remdesivir. None received tocilizumab. Median hospital LOS was 10.33(+/- 5) days. None were discharged on anticoagulation, either for a new VTE event or as extended thromboprophylaxis. All were discharged home without supplemental oxygen, but with pulmonary medicine and primary care outpatient follow-ups.
3. Characteristics and outcomes of hospitalized patients for Pulmonary Embolism
Median time from positive COVID-19 test to diagnosis of VTE was 151(± 122) days. Only three (13%) patients were diagnosed with VTE as outpatients. Most (88%) presented to the emergency room with primary complaints of dyspnea (96%), chest pain (21%), leg swelling (17%), and cough (17%). All inpatients had elevated D-dimer levels. Three (13%) were on therapeutic anticoagulation at the time of VTE diagnosis. All patients underwent a CTA, with a central PE (in the main pulmonary or lobar artery) in seven (33%). Eight patients (31%) had elevated cardiac biomarkers suggestive of right ventricular dysfunction. Three (14.2%) had elevated troponin, one (4.8%) had elevated Brain Natriuretic Peptide (BNP) levels alone, with one (4.8%) patient with elevations in both. Venous lactate was elevated in five patients (24%). Based on the 2019 European Society of Cardiology Guidelines on Diagnosis and Management of Acute PE, most patients were either classified as low-risk (52.3%), or intermediate-low risk (38.1%) for mortality. Five (24%) patients concomitantly had a proximal Deep Venous Thrombosis (DVT) of the lower extremity on ultrasonography. All received systemic anticoagulation with Low Molecular Weight Heparin (48%), Unfractionated Heparin (38%), or Direct-Acting Oral Anticoagulants (DOAC) (14%). None required advanced reperfusion therapies nor received IVC filter placement. No major bleeding (as defined by the International Society on Thrombosis and Haemostasis) was noted during hospital stay, with two patients (10%) developing minor bleeding that did not necessitate discontinuation of therapeutic anticoagulation. All survived to hospital discharge. Presence of circulating IgG anti- SARS-CoV-2 was noted in all patients.
4. Thrombophilia testing
Of twenty-one patients requiring admission for PE, twenty (95%) presented for a follow-up at our pulmonary medicine clinic. Three (12.5%) were diagnosed with VTE as outpatients and continued to follow up at our clinic. They were evaluated for OSA utilizing a validated standard clinical questionnaire (STOP-BANG) as well as thrombophilia testing. The most common hypercoagulable state detected was elevated factor VIII levels in almost half the population (46%). A positive lupus anticoagulant (LA) assay and indeterminate-high levels of anti-cardiolipin (aCL) IgM levels were detected in five (29%) patients each. Two patients (12%) were diagnosed with Protein C deficiency. Prothrombin (factor II) G20210A variant was noted in two (11%).
All patients documented adherence to the recommended therapeutic anticoagulation regimen. Most were prescribed a DOAC (96%), with one on Coumadin. Most continued to remain symptomatic, with dyspnea out of proportion from baseline being the most common complaint (96%), followed by fatigue (30%), chest pain (43%), cough (22%), and dizziness (22%).
Table 1: Characteristics of Patients Diagnosed with COVID-19.
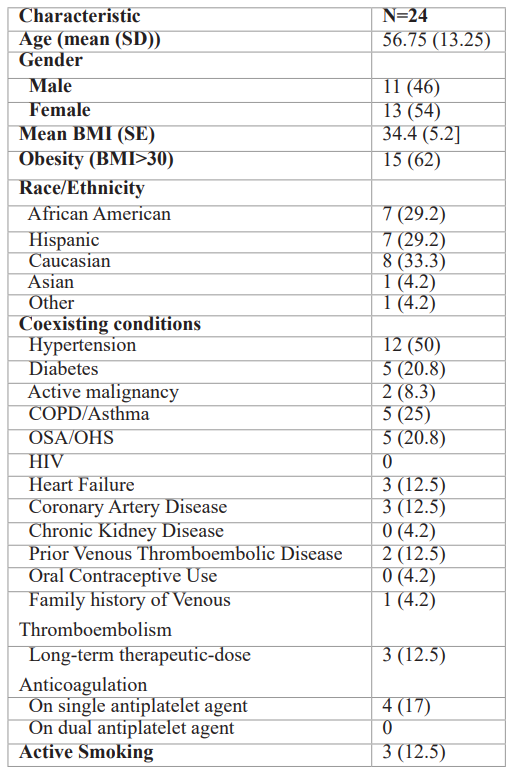
Note – except where indicated, data are number of patients, with percentages in parentheses. BMI=body mass index; HIV=human immunodeficiency virus; COPD=chronic obstructive pulmonary disease; OSA=obstructive sleep apnea; OHS=obesity hypoventilation syndrome.
Table 2: Characteristics of Patients Admitted for COVID-19.
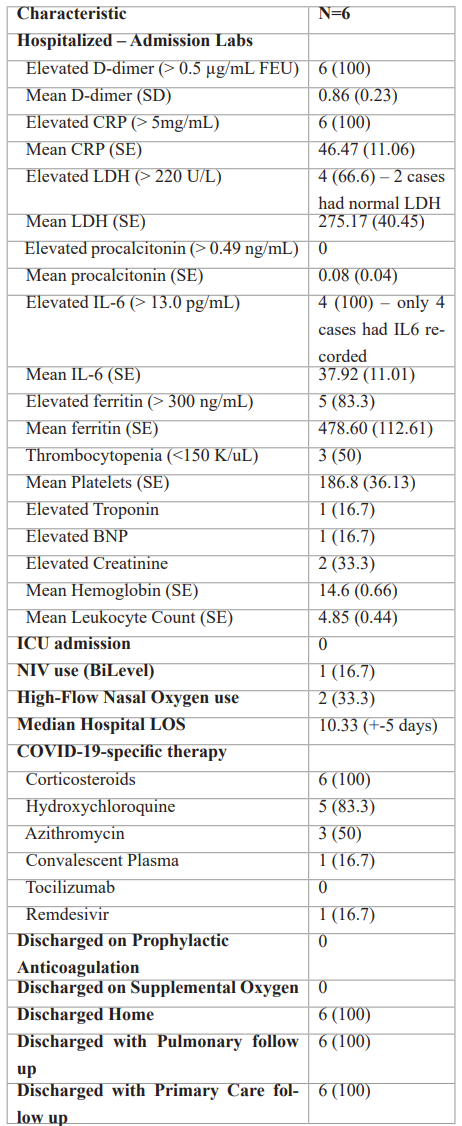
Note – except where indicated, data are number of patients, with percentages in parentheses. CRP=C-reactive protein; LDH=lactate dehydrogenase; IL-6=interleukin-6; BNP=brain natriuretic peptide; ICU=intensive care unit; NIV=non-invasive ventilation; LOS=length of stay.
Table 3: Hospital Admission for Acute Pulmonary Embolism (PE).
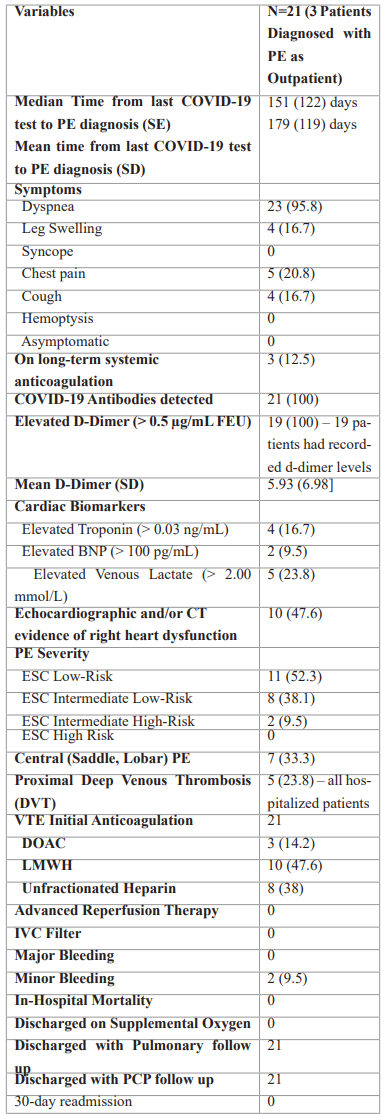
Note – except where indicated, data are number of patients, with percentages in parentheses. BNP=brain natriuretic peptide; ESC=European Society of Cardiology; PE=pulmonary embolism; VTE=venous thromboembolism; DOAC=direct oral anticoagulant; LMWH=low molecular weight heparin; IVC=inferior vena cava; PCP=primary care physician.
Table 4: Outpatient Hypercoagulability Work-up.
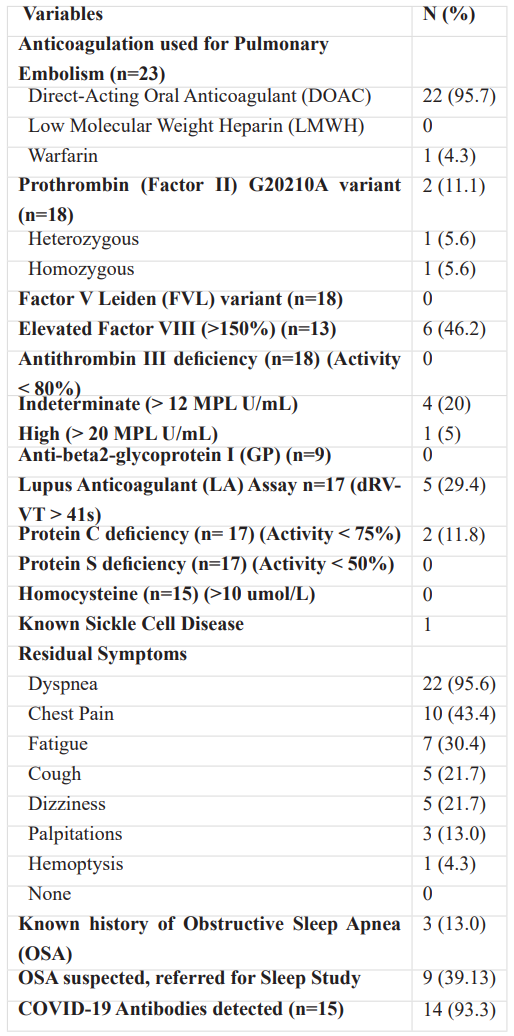
Note – except where indicated, data are number of patients, with percentages in parentheses.
Discussion
We present a series of 24 patients who were diagnosed with a symptomatic PE with mean of 179 days after the COVID-19 diagnosis. Although VTE is a common complication of COVID-19 during hospitalization, particularly in patients with critical illness [11], 75% of patients in this series were treated for COVID-19 as outpatients. The mean (179(+/-119 days)) and median (151 days) time of diagnosis of PE following COVID-19 was much later than previously described [19-21]. All patients were symptomatic with the majority with typical PE presentations of dyspnea/chest pain. Though none had a life-threatening PE requiring advanced therapy, 23% had an elevated lactate on admission, proximal DVT, and 33% had a central PE. The mean D-dimer was 5.93 [SD 6.98]. An increased level is an independent predictor of PE and critical illness and mortality in hospitalized patients with COVID-19 [23-24]. In a study of 150 patients, 25.3% had a persistent elevation up to 4 months after COVID-19 diagnosis, of whom 81 were managed as outpatients. More commonly elevated in hospitalized, older patients, the mean level was 744 ng/ml [25].
1. Risk factors for developing PE
Many study patients had risk factors predisposing to PE including obesity, OSA/OHS, malignancy, and thrombophilia serologies including an elevated Factor VIII level (46.2%), IgM anticardiolipin antibodies (25%), and a lupus anticoagulant (29%). COVID-19 infection induces inflammatory damage to the endothelial cells, causing an increase in coagulation biomarkers including Factor VIII, vWF, fibrinogen and selectin [26]. High Factor VIII levels have been associated with thrombosis including PE in patients with severe COVID-19 [27]. Mechanistically, SARS-CoV-2 has been demonstrated to bind to CLEC4M receptor that is involved in vWF and Factor VIII clearance. It is hypothesized that competitive binding of SARS-CoV-2 to CLEC4M could be responsible for a decreased clearance, promoting a hypercoagulable state [28]. Remarkably, Factor VIII levels were elevated in patients many months after COVID-19. Lupus Anticoagulant positivity in patients hospitalized with COVID-19 is associated with an increase in arterial and venous thrombosis [29].
2. Persistent symptoms following mild COVID-19, and risk for PE
Persistent symptoms of dyspnea and chest pain were prevalent on outpatient follow-up following PE. We suggest that “post PE syndrome” [30] may not be uncommon in patients with a PE following COVID-19. Deconditioning as a cause of post PE dyspnea has been inferred from CPET studies in patients following PE, which may have contributed to dyspnea and fatigue in our cohort [31]. We did not perform RV function or perfusion imaging studies to determine the causes of post PE dyspnea. Although persistent pulmonary vascular obstruction after PE is common [32], it may not be associated with significant physiological abnormalities of the pulmonary vasculature [33].
We emphasize that 75% of our patients with post-COVID-19 PE did not suffer severe COVID-19 illness or require hospital admission. This is in contrast from the well-documented increased risk of VTE in critically ill COVID-19 patients requiring hospitalization [10,11], who have been described to be at a higher risk of PE compared to historical controls with respiratory failure from a non-COVID-19 viral infection [34]. We therefore recommend one to consider PE as a cause for dyspnea or chest pain, even many weeks after the COVID-19 diagnosis, particularly if VTE risk factors are present with elevated D-dimer.
Many COVID-19 survivors experience “Long COVID” - new or chronic symptoms affecting multiple organ systems, lasting weeks to months. Symptoms can occur in patients who have had a mild or severe COVID-19 [35]. In a recent survey for long COVID of 3762 participants, over 90% and 80% reported respiratory symptoms and dyspnea, respectively [36]. To date, PE has not been emphasized as a possible cause of respiratory symptoms many weeks after COVID-19.
The need for thromboprophylaxis after discharge following admission for COVID-19 is controversial [12-14]. Studies have described an increased risk of post-discharge VTE following hospitalization for high risk non-COVID-19 medical illness including sepsis and pneumonia within 6 weeks of discharge [19]. Prophylactic anticoagulation reduces the risk of VTE in acutely ill, hospitalized patients [37,38], at a cost of an increased risk of bleeding [39]. A post-COVID VTE rate of 0.48% within 42 days post-discharge which was not significantly higher than the rate following hospitalization after a medical illness has been described [20]. There was a similarly low rate of 0.6% within 30 days of discharge following hospitalization for COVID-19 [21]. In contrast, VTE rates of 1.55% were described among 4906 patients followed for 90 days after discharge following hospitalization for COVID-19, of whom 13.2% received post-discharge thromboprophylaxis [40]. Multivariate analysis identified VTE risk factors similarly identified in our study, including a personal/family history of VTE and laboratory abnormalities associated with thrombophilia. Additional risk factors described included a history of cancer and an IMPROVE-DD VTE score≥4 [40].
Per ACTIV-4B randomized trial, there is no evidence supporting aspirin or DOAC use to reduce subsequent VTE in symptomatic clinically stable outpatients who do not require hospitalization [41]. Therefore, providing thromboprophylaxis to our study patients at the time of their COVID-19 diagnosis would not have reduced the risk of developing thromboembolism. There are ongoing randomized trials addressing the potential efficacy and safety of antithrombotic therapy to reduce VTE following outpatient management of COVID-19 [42]. In view of the lower rate of thrombotic events than anticipated in ambulatory treated COVID-19 patients in the ACTIV-4B study, future studies with adequate power evaluating potential benefits of prophylactic anticoagulation in COVID-19 patients treated as outpatients are required [41].
3. Limitations
Our study has a number of possible limitations. Firstly, we acknowledge that we cannot prove causality between COVID-19 infection and the incidence of PE. However, a significant number of our patients had evidence of persistent thrombophilic state as manifested by elevated Factor VIII and positive lupus anticoagulant. Persistent elevation of D-dimer has been described in patients post COVID-19 after a median of 3 months [46], and was elevated in 25.3% patients four months after COVID-19 infection, where 29% of this cohort were treated as outpatients [25]. A chronic endotheliopathy has been described in convalescent patients post COVID-19, where elevated levels of biomarkers of endothelial cell activation, including Factor VIII and von Willebrand factor antigen (VWF:aAg) levels are increased compared to healthy controls [47]. Please note that there was absence of alternative risk factors predisposing to PE in our study cohort including recent surgery, former active cancer, or prolonged immobilizing. Moreover, we did not systematically follow up all patients diagnosed with COVID-19 as inpatients or outpatients. Since the study period preceded the surge from the potent delta variant, an underestimation of late PE presentation following COVID-19 is possible [43]. However, subsequent to the completion of this study, a significant number of patients received vaccination against COVID-19 and have had access to monoclonal antibody therapy [44], which may lead to a lower severity of disease with lower risk of PE. Post-COVID-19 VTE rate may also have been underestimated as many with VTE following hospital discharge are asymptomatic [45]. Of note, most study patients with late presentation of PE had COVID-19 were treated exclusively in the outpatient setting. We are unaware how many outpatients received any kind of VTE prophylaxis at the time of their COVID-19 diagnosis.
Conclusion
We present 24 patients who presented with an acute PE many weeks after experiencing acute COVID-19, of whom 75% did not require hospitalization for COVID-19. It has not yet been determined that prophylactic administration of anticoagulation for outpatient treated COVID-19 can decrease the subsequent development of VTE. We recommend that patients being evaluated for acute symptoms of shortness of breath or chest pain who were diagnosed with COVID-19 even many weeks previously should be considered to have a possible PE, particularly if the D-dimer or Factor VIII are elevated, and if the patient has clinical risk factors for VTE. A sustained elevation of D-dimer post COVID-19 may have implications on the clinical management of these patients.
Key points
- This case series describe twenty-four patients presenting with an acute PE many weeks after the SARS-CoV-2 infection, where the majority had COVID-19 treated exclusively in the outpatient setting.
- All patients had typical presentations of PE such as dyspnea, chest pain.
- D-dimer or Factor VIII levels, clinical risk factors for VTE.
- Clinicians should consider PE as a diagnosis in patients with relevant symptoms and predisposing risk factors with diagnosis of COVID-19 weeks earlier, including those previously managed as outpatients.
- Studies are required to evaluate the efficacy and safety of thromboprophylaxis in patients diagnosed with COVID-19 as outpatients.
Statements and Declarations: N/A
All authors stated above hereby declare no conflicts of interest.
All authors stated above have no financial/nonfinancial disclosures pertaining to this study.
Names of collaborators: N/A
Financial/nonfinancial disclosures: All authors have no financial/nonfinancial disclosures pertaining to this study.
References
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The lancet, 2020; 395(10224): 565-74.
- Osuchowski MF, Winkler MS, Skirecki T, Cajander S, Shankar-Hari M, Lachmann G, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. The Lancet Respiratory Medicine, 2021; 9(6): 622-642.
- Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine, 2021; 38: 101019.
- Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis, 2020; 18(6): 1421-1424.
- Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thrombosis research, 2020; 190: 62.
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of thrombosis and haemostasis, 2020; 18(4): 844.
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. New England Journal of Medicine, 2020; 383(2): 120-128.
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. The Lancet, 2020; 395(10234): 1417-1418.
- Di Minno A, Ambrosino P, Calcaterra I, Di Minno MN. COVID-19 and venous thromboembolism: a meta-analysis of literature studies. InSeminars in thrombosis and hemostasis. Thieme Medical Publishers, 2020; 46(07): pp. 763-771.
- Obi AT, Barnes GD, Napolitano LM, Henke PK, Wakefield TW. Venous thrombosis epidemiology, pathophysiology, and anticoagulant therapies and trials in severe acute respiratory syndrome coronavirus 2 infection. Journal of Vascular Surgery: Venous and Lymphatic Disorders, 2021; 9(1): 23-35.
- Klok FA, Kruip MJ, Van der Meer NJ, Arbous MS, Gommers DA, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis research, 2020; 191: 145-147.
- Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest, 2020; 158(3): 1143-1163.
- Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. Journal of the American college of cardiology, 2020; 75(23): 2950-2973.
- Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. Journal of thrombosis and thrombolysis, 2020; 50(1): 72-81.
- Connors JM, Brooks MM, Sciurba FC, Krishnan JA, Bledsoe JR, Kindzelski A, et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. Jama, 2021; 326(17): 1703-1712.
- Di Tano G, Moschini L, Loffi M, Testa S, Danzi GB. Late pulmonary embolism after COVID-19 pneumonia despite adequate rivaroxaban treatment. European Journal of Case Reports in Internal Medicine, 2020; 7(7).
- Vlachou M, Drebes A, Candilio L, Weeraman D, Mir N, Murch N, et al. Pulmonary thrombosis in Covid-19: before, during and after hospital admission. Journal of thrombosis and thrombolysis, 2021; 51(4): 978-984.
- Taha M, Nguyen P, Sharma A, Taha M, Samavati L. Forty-One-Year-Old Man with pulmonary embolism 5 months after COVID-19. Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine, 2021; 15: 1179548420986659.
- Spyropoulos AC, Ageno W, Albers GW, Elliott CG, Halperin JL, Hiatt WR, et al. Post-discharge prophylaxis with rivaroxaban reduces fatal and major thromboembolic events in medically ill patients. Journal of the American College of Cardiology, 2020; 75(25): 3140-3147.
- Roberts LN, Whyte MB, Georgiou L, Giron G, Czuprynska J, Rea C, et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood, 2020; 136(11): 1347-1350.
- Patell R, Bogue T, Koshy A, Bindal P, Merrill M, Aird WC, et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood, 2020; 136(11): 1342-1346.
- Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). European heart journal, 2020; 41(4): 543-603.
- Mestre-Gómez B, Lorente-Ramos RM, Rogado J, Franco-Moreno A, Obispo B, Salazar-Chiriboga D, et al. Incidence of pulmonary embolism in non-critically ill COVID-19 patients. Predicting factors for a challenging diagnosis. Journal of thrombosis and thrombolysis, 2021; 51(1): 40-46.
- Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JC, Fogerty AE, Waheed A, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood, 2020; 136(4): 489-500.
- Townsend L, Fogarty H, Dyer A, Martin‐Loeches I, Bannan C, Nadarajan P, et al. Prolonged elevation of D‐dimer levels in convalescent COVID‐19 patients is independent of the acute phase response. Journal of Thrombosis and Haemostasis, 2021; 19(4): 1064-1070.
- Lowenstein CJ, Solomon SD. Severe COVID-19 is a microvascular disease. Circulation, 2020; 142(17): 1609-1611.
- Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive care medicine, 2020; 46(6): 1089-1098.
- Alam W. Hypercoagulability in COVID-19: A review of the potential mechanisms underlying clotting disorders. SAGE open medicine, 2021; 9: 20503121211002996.
- Gil MR, Barouqa M, Szymanski J, Gonzalez-Lugo JD, Rahman S, Billett HH. Assessment of lupus anticoagulant positivity in patients with coronavirus disease 2019 (COVID-19). JAMA network open, 2020; 3(8): e2017539.
- Pugliese SC, Kawut SM. The post–pulmonary embolism syndrome: real or ruse? Annals of the American Thoracic Society, 2019; 16(7): 811-814.
- Kahn SR, Hirsch AM, Akaberi A, Hernandez P, Anderson DR, Wells PS, et al. Functional and exercise limitations after a first episode of pulmonary embolism: results of the ELOPE prospective cohort study. Chest, 2017; 151(5): 1058-1068.
- Planquette B, Ferré A, Peron J, Vial-Dupuy A, Pastre J, Mourin G, et al. Residual pulmonary vascular obstruction and recurrence after acute pulmonary embolism. A single center cohort study. Thrombosis research, 2016; 148: 70-75.
- Albaghdadi MS, Dudzinski DM, Giordano N, Kabrhel C, Ghoshhajra B, Jaff MR, et al. Cardiopulmonary exercise testing in patients following massive and submassive pulmonary embolism. Journal of the American Heart Association, 2018; 7(5): e006841.
- Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation, 2020; 142(2): 184-186.
- “Post-Covid Conditions.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention.
- Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine, 2021; 38: 101019.
- Samama MM, Cohen AT, Darmon JY, Desjardins L, Eldor A, Janbon C, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. New England Journal of Medicine, 1999; 341(11): 793-800.
- Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation, 2004; 110(7): 874-879.
- Dentali F, Mumoli N, Prisco D, Fontanella A, Di Minno MN. Efficacy and safety of extended thromboprophylaxis for medically ill patients. Thrombosis and Haemostasis, 2017; 117(03): 606-617.
- Giannis D, Allen SL, Tsang J, Flint S, Pinhasov T, Williams S, et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood, 2021; 137(20): 2838-2847.
- Connors JM, Brooks MM, Sciurba FC, Krishnan JA, Bledsoe JR, Kindzelski A, et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. Jama, 2021; 326(17): 1703-1712.
- Talasaz AH, Sadeghipour P, Kakavand H, Aghakouchakzadeh M, Kordzadeh-Kermani E, Van Tassell BW, et al. Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC state-of-the-art review. Journal of the American College of Cardiology, 2021; 77(15): 1903-1921.
- Bager P, Wohlfahrt J, Rasmussen M, Albertsen M, Krause T. Hospitalisation associated with SARS-CoV-2 delta variant in Denmark. The Lancet. Infectious Diseases, 2021.
- S. Department of Health and Human Services. “Anti-SARS-cov-2 monoclonal antibodies”. National Institutes of Health.
- Guyatt GH, Eikelboom JW, Gould MK, Garcia DA, Crowther M, Murad MH, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest, 2012; 141(2): e185S-94S.
- Lehmann A, Prosch H, Zehetmayer S, Gysan MR, Bernitzky D, Vonbank K, et al. Impact of persistent D-dimer elevation following recovery from COVID-19. PLoS One, 2021; 16(10): e0258351.
- Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E, et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. Journal of thrombosis and haemostasis, 2021; 19(10): 2546-2553.

