The Outpatient Management of Non-Server Forms of Covid-19
Bel Houari M1,*, Zalaghi R1, Bouhlala N1,2, Zhim I1,2, Bousliman Y3 and Tadlaoui Y1,2
1Pharmacy department of the Mohammed V Military Training Hospital in Rabat, Morocco
2Department of Therapeutic Chemistry and Clinical Pharmacy of the Faculty of Medicine and Pharmacy of Rabat - Mohammed V University of Rabat, Morocco
3Department of Toxicology of the Faculty of Medicine and Pharmacy of Rabat - Mohammed V University of Rabat, Morocco
Received Date: 07/08/2023; Published Date: 22/12/2023
*Corresponding author: Meriem Bel Houari, Pharmacy department of the Mohammed V Military Training Hospital in Rabat, Morocco
Abstract
On December 8th 2019, an infectious disease in the form of idiopathic pneumonia emerged in Wuhan, and on March 11th 2020, COVID-19 was declared a pandemic by the WHO due to its unprecedented infectivity and pathogenicity. Subsequently, several clinical trials and diagnostic and therapeutic strategies against SARS-CoV-2 were rapidly developed, with varying degrees of usefulness in managing the disease.
This prospective study aimed to assess the outpatient management of confirmed COVID-19 patients by collecting data from individuals who were not hospitalized. The study spanned six months, during which epidemiological, clinical, paraclinical, and therapeutic data were gathered from 511 participants, of whom 311 were confirmed COVID-19 cases. The majority of patients were females (64%), with an average age of 28 years. PCR testing was the most commonly used confirmation method (54%), followed by rapid testing (27%) and serological testing (3.50%). Remarkably, 90% of the studied patients were vaccinated against COVID-19.
The most frequently reported symptoms by patients included fatigue, muscle and joint pains, headaches, fever, and loss of smell or taste. Regarding the therapeutic approach, 57.6% of patients strictly followed the national treatment protocol, while others opted for alternative treatments or refused to adhere to the recommended protocol. Some patients used antibiotics, corticosteroids, or the drug Molnupiravir. Approximately 97 patients reported adverse effects related to the treatment.
This study highlights the importance of an individualized approach in managing COVID-19 patients and sheds light on significant aspects of outpatient care and the value of adhering to recommended therapeutic protocols.
Keywords: COVID-19; Diagnostic; Treatment; Therapy
Introduction
On December 8th 2019, cases of idiopathic pneumonia emerged in Wuhan, central China. A month later, the causative agent was identified as severe acute respiratory syndrome coronavirus 2, first named 2019-nCoV by the International Committee on Taxonomy of Viruses (ICTV), for its similarity in genomic sequence to coronavirus responsible for the SARS epidemic in 2003 [1].
The first case in Morocco was confirmed on March 2nd 2020. On March 11th 2020, COVID-19 was declared a pandemic by the WHO due to its speed and extent of transmission [2].
Given its unprecedented infectivity and pathogenicity, indeed, its clinical management has represented the greatest challenge and strained our medical and public health facilities [3].
This has led to the development and rapid testing of several treatments against SARS-CoV-2, at an exceptional rate, as well as the establishment of many therapeutic strategies that have proven to be more or less useful in the management of the disease. COVID-19 disease [2].
For this study, we will be particularly interested in patients who have developed a non-severe form of the disease, starting with the diagnostic stage through the treatment they have undergone on an outpatient basis in order to observe, analyze and discuss the in charge of these positive COVID-19 patients in accordance with the international and national protocols adopted in the management of the coronavirus crisis.
Materials and Methods
This is an analytical and descriptive cross-sectional study carried out with confirmed COVID-19 positive patients, treated in an outpatient setting and not hospitalized. It was spread over a period of 6 months from April 6th 2022 to October 20th 2022. The data was collected via an electronic questionnaire on the google Forms platform distributed on social networks. The questionnaires were completed anonymously with the consent of the respondent and respecting the confidentiality of the information.
The questionnaire has 4 items:
-Item I: Epidemiological profile
-Item II: Clinical and paraclinical profile
-Item III: Therapeutic profile
Results
A total of 511 people responded to our questionnaire, including 60.9% or 311 confirmed positive COVID-19 cases.
Women represented 199 cases, or 64% of our population, and men 112 cases, or 36%, with a sex ratio of 0.56.
The average age of the population was 28 years old. The most represented age group was that of 18 to 24 years with a percentage of 38% against the group of patients aged 50 to 64 years does not exceed 14.47% of respondents.
RT-PCR was the most used confirmation test with a percentage of 54%, followed by rapid test at 27%, serological test at 3.50% and chest X-ray in 4.50% of patients.
Of the entire population surveyed and positive (n = 311) 90% are vaccinated against COVID-19.
In our study, 294 (94.50%) people showed symptoms while 17 (5.50%) claimed to have shown no symptoms. The most frequently cited symptoms were asthenia (n=227), muscle and joint pain (n=199), headache (n=196), fever (n=170), anosmia and/or ageusia (Figure N°1).
Our study revealed that 265 people or 85.2% have knowledge of the national therapeutic protocol while 14.8% ignore it.
A majority of 54.7% consulted and started their treatment following a medical prescription. Among the 45.3% who resorted to self-medication, 23.8% relied on research done on social networks and the internet to choose the drugs to take. The other 21.50% patients asked their pharmacist for advice on the treatment to follow.
The majority of cases, 57.6% of our study, strictly followed the national therapeutic protocol against 132 cases (42.4%) (Figure N°2).
Regarding the 132 patients who preferred not to follow the national protocol:
43.18% of cases followed the national therapeutic protocol without hydroxychloroquine,
25% of cases used only antipyretics and vitamins and minerals as treatment.
14 cases or 10.60% did not receive any treatment.
For the remaining 21.21% of cases, other treatments were considered.
59, or 19%, started their antibiotic therapy for the treatment of COVID-19, including 31 cases in the first 5 days of infection while 28 cases after the second week.
Only 7 cases or 2.30% resorted to the use of Molnupiravir.
In our study series, 52 cases (16.8%) had recourse to corticosteroid therapy. 55.76% of them took it within the first 5 days and 44.26% after 7 days.
97 patients answered YES. The list of adverse effects encountered is summarized in (Figure N°3).
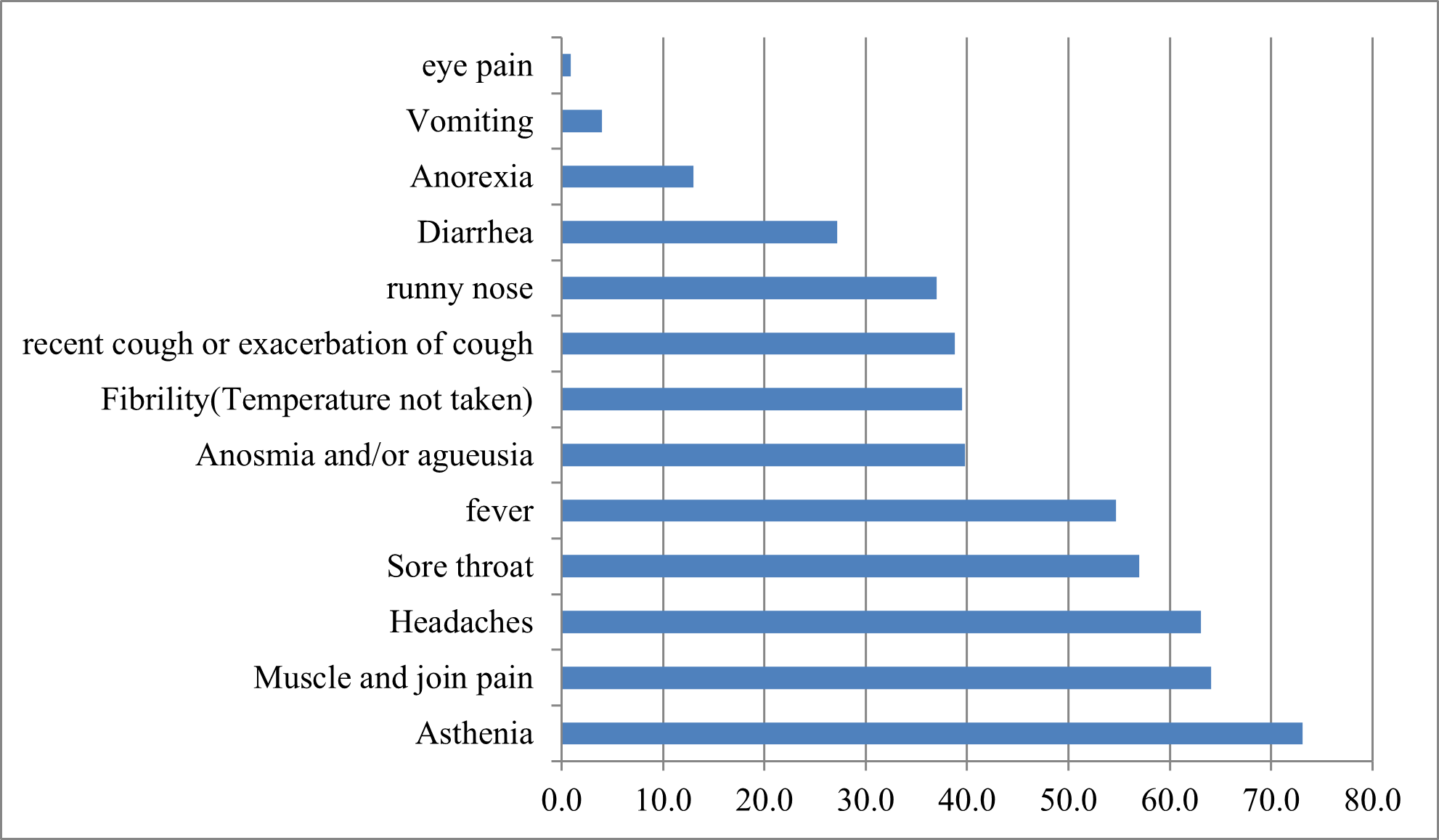
Figure N°1 : Main symptoms experienced by patients with Covid-19.
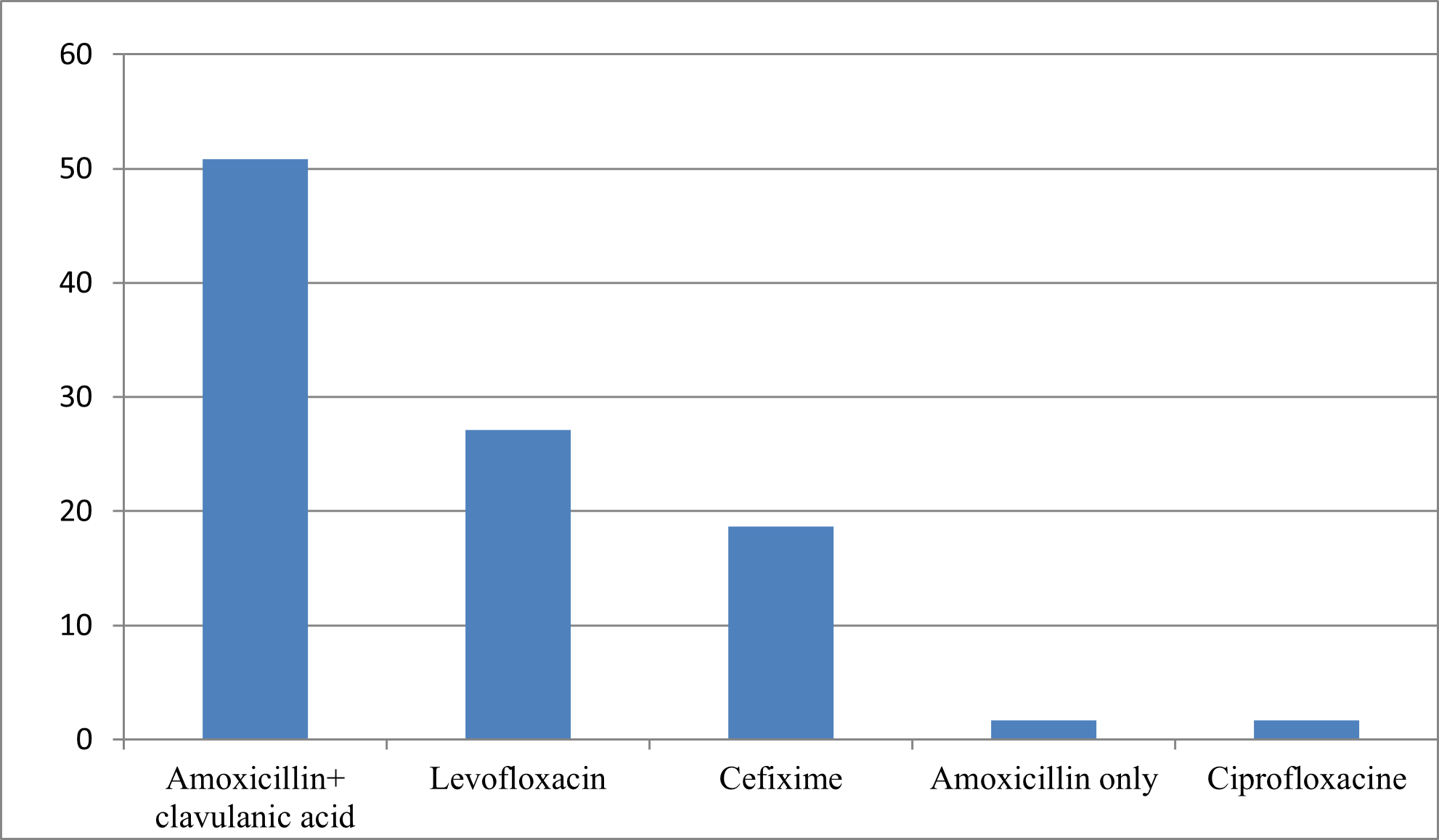
Figure N°2 : Main antibiotic treatments (or azithromycin) used by patients with Covid-19.
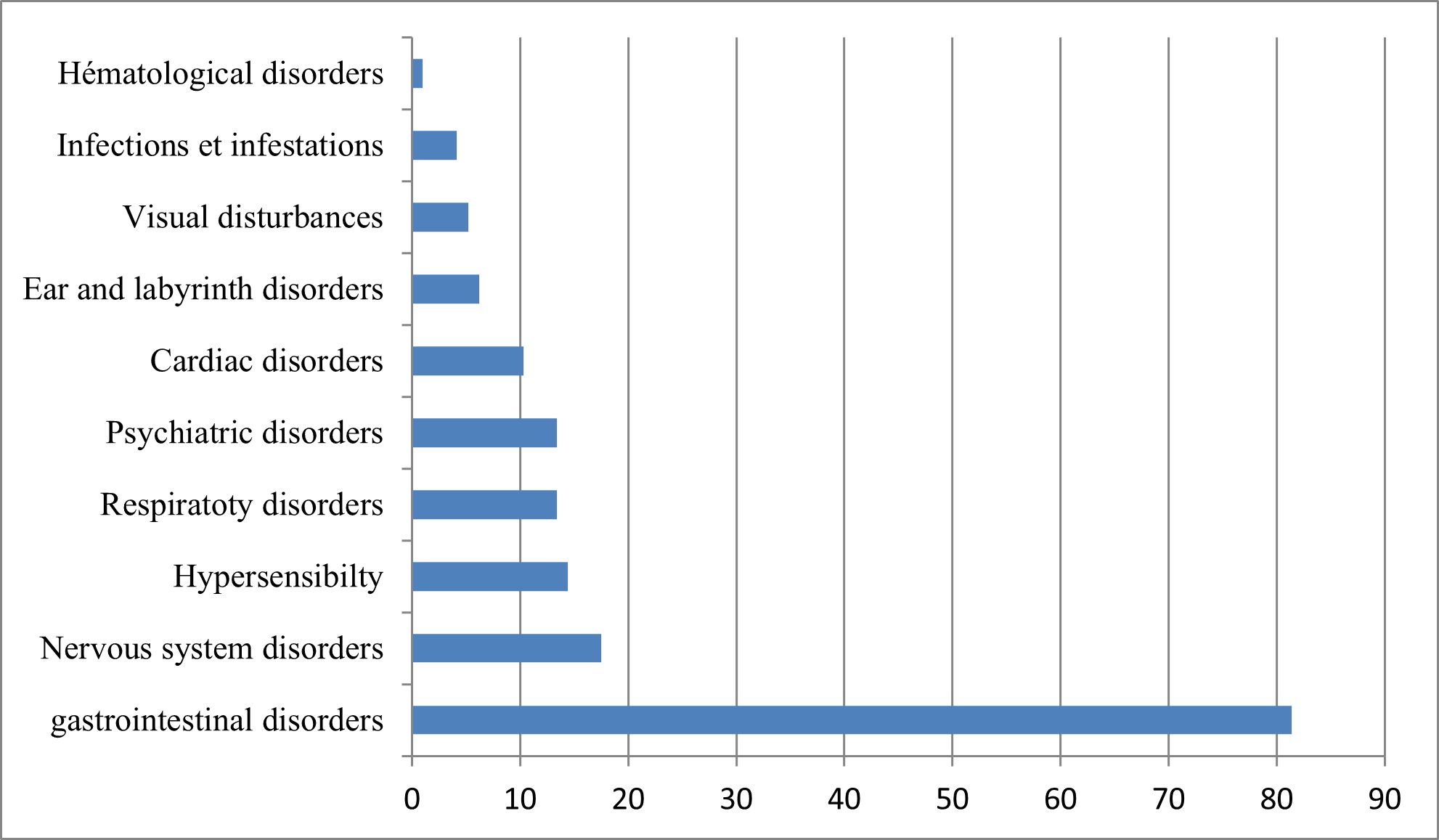
Figure N° 3 : Classification of the main adverse effects reported (in order of frequency).
Discussion
Of the studied sample of 511 cases, 311 have already contracted the COVID-19 virus, or 60.9%.
It is estimated that without a vaccine, herd immunity is achieved when approximately 70% of the population is infected [4].
According to a study carried out on the Moroccan city of Agadir, men represented 46% of our population and women 54%, this is consistent with the results obtained [5].
PCR has been the most commonly used diagnostic test, it remains the reference technique, providing definitive diagnosis by detecting the SARS-CoV-2 viral genome in biological samples [6]. It is particularly useful during the acute phase of viral infection, when viral replication is active, its effectiveness decreases as the levels of antibodies produced by the body increase [7].
Rapid screening tests are an alternative to RT-PCR for rapid clinical and quarantine decisions. These are specific tests but very insensitive because they strongly depend on the patient's viral load [8]. This low sensitivity therefore constitutes a major obstacle for use aimed at detecting all suspected COVID-19 cases.
Chest x-rays play an important role in the diagnosis, monitoring and assessment of disease severity. Given the typical presentation of the lesion, it has been used as a diagnostic tool with a sensitivity as high as 98%. However, early in the disease, 15–20% of symptomatic patients may be normal [9].
Serological tests are a very sensitive (99.9%) and specific (100%) technique beyond 21 days after the onset of symptoms. Currently, they have their place in the epidemiological surveillance of the disease, and in the diagnostic strategy, in addition to the virological test by RT-PCR [10].
In our study series, a majority of 90% of cases were vaccinated. Morocco was, moreover, the first African country to launch a free vaccination campaign against the covid-19 virus, in January 2021. The number of vaccinated people has now reached around twenty-five million people for the first dose. And twenty-three million people received the booster dose.
Similarly, for the infected population, the possibility of being asymptomatic can vary, depending on the scenario and the population, from 5% to 80% [11]. A study conducted in China on 328 adults revealed that 5% of cases were asymptomatic, which is consistent with our study [12].
As confirmed by other similar studies, clinical manifestations during mild COVID-19 are generally nonspecific, mainly general signs and ENT signs, the most reported symptoms were general signs (87.9%), ENT signs (78.8%), arthromyalgia (72.7%), anosmia (from 30 to 70.6%), taste disorders (from 30 to 63.3%), headaches (69.5%), abdominal signs (39.8%), chest pain (38.4%) and dry cough (32.6%) [13].
54.7% of patients followed a medical prescription following a medical consultation 45.3% resorted to self-medication, in fact, a recent study carried out in Togo showed that approximately one in three people had already used to this practice to prevent Covid-19 [14]. Another study carried out in Bangladesh revealed that the prevalence of self-medication during the Covid-19 epidemic was 88.33% [15]. In Kenya, the prevalence of self-medication increased from 36.2% before the pandemic to 60.4% during the pandemic [16]. Among the reasons that may explain this, the avoidance of medical structures as well as other socio-economic factors. Faced with this situation, many substances have been used without medical advice. The main source of information was social networks, followed by advice to community pharmacists [17].
A technical and scientific advisory committee has been set up by the Moroccan Ministry of Health, in order to develop protocols and adapt them, continuously, according to the epidemiological evolution of the health crisis. Its first protocol dates back to March 23th 2020 and the last update dates from August 04th 2022, with the addition of Molnupiravir which is used for people with risk factors [18].
The use of the combination Azithromycin + Hydroxychloroquine has been the most used, however, data from randomized controlled trials evaluating the use of hydroxychloroquine with or without azithromycin in hospitalized patients have not demonstrated an improvement in the clinical status or overall mortality compared to placebo [19,20].
Hydroxychloroquine was reported out of a total of 2814 times in relation to covid-19 in VigiBase. Of these 2814 reports, 114 cases concerned the sole use of this molecule and in the remaining cases it was an association mainly with azithromycin which itself counted a total of 1784 reports [21].
Among the most common side effects (more than 10% of cases) are abdominal pain and nausea. 1–10% of cases noted the onset of diarrhea, vomiting, loss of appetite, headache, itching, rash, and accommodation disturbance [22,23].
These data are consistent with our results, which may explain the suspicion of the incrimination of this molecule in the appearance of these adverse effects in the subjects who administered it.
Patients receiving these treatments concomitantly are exposed to possible prolongations of the corrected QT (QTc) interval of the surface electrocardiogram. The cardiac toxicity of hydroxychloroquine is dose-dependent and cases of serious arrhythmias have been reported during overdose but also at therapeutic doses [24].
In the WHO guideline published on 10/06/2022, it was strongly recommended against the use of hydroxychloroquine regardless of the severity of COVID-19 disease [25].
Administration of multiple doses of azithromycin has been implicated in the appearance of digestive signs: diarrhea/loose stools (4-5%), abdominal pain (2-3%), vomiting (1%) and nausea (3- 4%) [26]. A percentage of 10.30% of cardiac manifestations was also noted, which can be explained by the fact that azithromycin was responsible for the cases of prolongation of cardiac repolarization and prolongation of the QT interval implying a risk of occurrence of cardiac arrhythmia and torsade de pointes. The use of azithromycin must be preceded by the realization of an ECG (electrocardiogram) in order to avoid any complication
Azithromycin can also cause superinfection like all antibiotics, hence the need for monitoring for signs of superinfection. In our study, 4.10% of cases showed infections and infestations.
Psychiatric, nervous system and ear and labyrinth disorders have also been reported with the use of this molecule as low frequency adverse effects (≥ 1/1,000 to < 1/100) [27].
Molnupiravir used by prescription in only 2.3%, is a direct-acting broad-spectrum oral antiviral agent acting on the RdRp enzyme by inhibiting it and thus causing several errors in the replication of the SARS-CoV-2 RNA virus [28]. Based on a meta-analysis of available Phase 1-3 studies, Molnupiravir was noted to demonstrate a significant reduction in hospitalizations and deaths in mild COVID-19 disease, when administered at most late 5 days after onset of symptoms [29]. Results of a double-blind, randomized, placebo-controlled Phase 3 trial reported that early treatment with Molnupiravir reduced the risk of hospitalization or death in unvaccinated at-risk adults with mild Covid-19 to moderate, laboratory confirmed [30].
Severe COVID-19 is associated with inflammation-related lung damage driven by the release of cytokines characterized by elevated inflammatory markers. At the start of the pandemic, the efficacy of glucocorticoids in patients with COVID-19 was not well described. The Covid-19 Therapy Evaluation Randomized Trial (RECOVERY), which included hospitalized patients with clinically suspected or laboratory-confirmed SARS-CoV-2 who were randomly assigned to receive dexamethasone (n=2104) or usual care (n=4321), showed that dexamethasone use resulted in lower 28-day mortality in patients on invasive mechanical ventilation or oxygen support, but not in patients receiving no support respiratory [19].
The 52 subjects of our study used corticosteroid therapy in their treatment without presenting the clinical and paraclinical signs which justify their indication, especially since 55.76% administered it during the viral phase of the infection, which is a contraindication. -absolute indication because corticosteroid therapy should never be started during the viral phase [31].
In our study, out of a total of 59 cases, 31 cases administered an antibiotic in the first 5 days, which constitutes a contraindication.
Antibiotic therapy is not necessary for a simple case of Covid-19 without severity or comorbidity criteria, bacterial co-infections being rare, this is in line with the results of the study conducted by Moretto et al, on the 222 patients included, 174 (78%) received antibiotic therapy. Amoxicillin–clavulanic acid accounted for 55% (95 patients) of prescriptions, followed by 3rd Generation Cephalosporins (25 patients, 30%). Piperacillin–tazobactam accounted for only 5% (9 patients) of first-line antibiotics. No bacterial co-infection has been documented [32].
The use of antibiotic therapy should not be systematic. In the context of COVID, it should only be administered in the following cases: [33].
Strong arguments for bacterial superinfection:
- Persistence of clinical signs and/or fever beyond 5 days;
- Purulent sputum;
- PCT > 2, or >1 and neutrophilic polynucleosis;
- Radiological appearance suggestive of bacterial infection;
- Isolation of one of several bacteria after a protected bronchial sample or after BAL.
Probabilistic treatment regimens are as follows:
- Non-serious pneumonia: Amoxicillin + Clavulanic Acid 1gx3/d PO;
- In case of allergy to beta-lactams, antipneumococcal fluoroquinolones can be used;
- The duration of treatment should not exceed 10 days and not less than 7 days.
The master of adverse effects was digestive manifestations with a percentage of 81.40%;
During this period, the consumption of antibiotics has increased significantly.
Antibiotic use can have several negative effects on the gut microbiota, including reduced species diversity, altered metabolic activity, and selection of antibiotic-resistant organisms, which can lead to diarrhea associated with antibiotics. antibiotics and recurrence of Clostridium difficile infections [34].
An increase in the consumption of vitamins and food supplements was noted by 91.4% of the pharmacists who participated in the study. In Togo, vitamin C and traditional medicines were also the most commonly used [14]. Vitamin D appears to help boost immunity [35]. Additionally, several studies have identified a link between seasonal respiratory infections and vitamin D deficiency [36, 37]. This has prompted several groups to propose vitamin D supplementation for the prevention and treatment of Covid-19[38]. However, given the lack of scientific evidence that high-dose vitamin D supplements help prevent or successfully treat Covid-19, experts caution against over-supplementation, especially if there is no medical supervision because of health risks, especially for renal function [39].
Research on the use of vitamin C for the treatment and prevention of respiratory infections is inconclusive [40]. A study in patients with sepsis and severe acute respiratory syndrome (SARS) failed to demonstrate the effectiveness of high-dose vitamin C infusions. In the context of Covid-19, a clinical study is underway in China [41]. However, vitamin C can cause kidney stones, so it should be used with caution in patients with a history of kidney stones or kidney failure [42].
Conclusion
This study conducted on the care of patients with COVID-19 on an outpatient basis highlights several key elements. She stresses the importance of implementing national recommended and up-to-date treatment protocols, as they remain the first choice for the majority of patients. However, the study also reveals that some patients opted for alternative treatments or refused to follow these recommendations. This raises concerns about informing and educating patients, as well as the need to better understand their motivations and choices. Providing patients with clear, evidence-based information is essential to help them make informed decisions about their treatment.
An individualized approach, taking into account patients' medical histories and underlying conditions, may improve outcomes and reduce complications.
Finally, this study highlights the essential role of vaccination in the prevention and management of COVID-19 and the fact that 90% of the patients studied were vaccinated and underlines the importance of continuing to promote vaccination and encourage the population to get vaccinated to avoid any serious or severe form of COVID-19.
Conflicts of Interest: Authors declares no conflicts of interest.
Grant Information: The author(s) received no specific funding for this work.
References
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical Features of Patients Infected With 2019 Novel Coronavirus In Wuhan, China. Lancet, 2020; 395(10223): 497–506.
- Dos Santos WG. Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomedicine & Pharmacotherapy, 2020; 129: 110493.
- Khan M, Adil SF, Alkhathlan HZ, Tahir MN, Saif S, Khan M, et al. COVID-19: A Global Challenge with Old History, Epidemiology and Progress So Far. Janv, 2021; 26(1): 39.
- Clemente-Suárez VJ, Hormeño-Holgado A, Jiménez M, Benitez-Agudelo JC, Navarro-Jiménez E, Perez-Palencia N, Maestre-Serrano R, et al. Dynamics of population immunity due to the herd effect in the COVID-19 pandemic. Vaccines, 2020; 8(2): 236.
- Daoui MA. Profil épidémiologique, clinique et biologique des patients COVID-19 hospitalisés au CHR Hassan II d'Agadir. Th Doc Méd, Marrakech, 2021: 156-p.
- Chan JF, Yip CC, To KK, Tang TH, Wong SC, Leung KH, et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens J ClinMicrobiol, 2020; 58(5): e00310-20.
- Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA, 2020. doi: 10.1001/jama.2020.8259.
- Castro R, Luz PM, Wakimoto MD, Veloso VG, Grinsztejn B, PerazzoH. COVID-19: a meta-analysis of diagnostic test accuracy of commercial assays registered in Brazil.Braz J Infect Dis, 2020; 24(2): 180-187.
- Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT findings in Coronavirus disease-19 (COVID-19): relationship to duration of infection Radiology, 2020; p. 200463.
- Krüttgen A, Cornelissen CG, Dreher M, Hornef M, Imöhl M, Kleines M. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J Clin Virol, 2020; 128: 104394.
- Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill, 2020; 25: 2000180.
- Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy, 2020; 75(7): 1730‑1741.
- Carrillo-Larco RM, Altez-Fernandez C. Anosmia and dysgeusia in COVID-19: A systematic review. Wellcome open research, 2020; 5.
- Sadio AJ, Gbeasor-Komlanvi AF, Konu RY, Bakoubayi AW, Tchankoni MK, BittyAnderson A, et al. Assessment of self-medication practices in the context of Covid-19 outbreak in Togo. Research Square (preprint version), 2020.
- Nasir M, Salauddin Chowdhury ASM, Zahan T. Self-medication during COVID-19 outbreak: a cross sectional online survey in Dhaka city. Int J Basic Clin Pharmacol, 2020; 9(9): 1325-1330. doi:10.18203/23192003.ijbcp20203522
- Onchonga D, Omwoyo J, Nyamamba D. Assessing the prevalence of self-medication among healthcare workers before and during the 2019 SARS-CoV-2 (COVID-19) pandemic in Kenya. Pharm. J., 2020; 28(10): 1149-1154. https://doi.org/10.1016/j.jsps.2020.08.003.
- Youssoufi K, Zoudani F. La communication des risques en temps de crise de Covid-19. Quelle contribution des acteurs de la santé face aux comportements à risque? Cas de l'automédication par la vitamine C. In Un monde de crises au prisme des communications organisationnelles, 2022.
- Avril V. Covid-19 et infection au SARS-CoV-2. :44.
- RECOVERY Collaborative Group, Horby P, Mafham M, Linsell L, Bell JL, Staplin N, et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med, 2020; 383(21): 2030-2040.
- Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med, 2020; 383(6): 517-525.
- Centre UM. About VigiBase, 2023.
- PLAQUENIL [Internet]. VIDAL, 2023.
- Coronavirus disease (COVID-19): Hydroxychloroquine, 2023.
- Chloroquine et Hydroxychloroquine: les points essentiels. RFCRPV, 2023.
- Therapeutics and COVID-19: Living guideline,
- pdf, 2023.
- Résumé des Caractéristiques du Produit, 2023.
- Pourkarim F, Pourtaghi-Anvarian S, Rezaee H. Molnupiravir: A new candidate for COVID- Treatment. Pharmacology Research & Perspectives, 2022; 10(1): e00909.
- Singh AK, Singh A, Singh R, Misra A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab Syndr, 2021; 15(6): 102329.
- Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med, 2022; 386(6): 509-520.
- Johns M, George S, Taburyanskaya M, Poon YK. A Review of the Evidence for Corticosteroids in COVID-19. Journal of pharmacy practice, 2022; 35(4): 626-637.
- Moretto F, Sixt T, Abdallahoui M, Devilliers H, Chavanet P, Catherine F, et al. Intérêt des antibiotiques au cours de la COVID-19. Médecine et Maladies Infectieuses, 2020; 50(6): S93.
- Adebisi YA, Jimoh ND, Ogunkola IO, Uwizeyimana T, Olayemi AH, Ukor NA, et al. The use of antibiotics in COVID-19 management: a rapid review of national treatment guidelines in 10 African countries. Tropical medicine and health, 2021; 49(1): 1-5.
- Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as Major Disruptors of Gut Microbiota. Front Cell Infect Microbiol, 2020; 10: 572912.
- Vanherwegen AS, Gysemans C, Mathieu C. Vitamin D endocrinology on the cross-road between immunity and metabolism. Mol CellEndocrinol, 2017; 453: 52–67. doi :10.1016/j.mce.2017.04.018.
- Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol, 2009; 158: 20–25. doi: 10.1111/j.13652249.2009.04001.x
- Zdrenghea MT, Makrinioti H, Bagacean C, Bush A, Johnston SL, Stanciu LA. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol, 2017; 27: 1–3. doi: 10.1002/rmv.1909.
- Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients, 2020; 12: E988. doi:10.3390/nu12040988.
- Lanham-New SA, Webb AR, Cashman KD, Buttriss JL, Falloweld JL, Masud T. Vitamin D and SARS-CoV-2virus/COVID- 19 disease. BMJ Nutr. Health, 2020; 0. doi:10.1136/bmjnph-000089.
- Gasmi A, Noor S, Tippairote T, Dadar M, Menzel A, Bjørklund G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin Immunol, 2020; 215: 108409. https://doi.org/10.1016/j.clim.2020.108409.
- Lotfi M, Hamblin MR, Rezaei N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin. Chim. Acta., 2020; 508: 254-266. doi: 10.1016/.j.cca.2020.05.044. 21.
- Liu F, Zhu Y, Zhang J, Li Y, Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ Open, 2020; 10: e039519. doi:10.1136/bmjopen-2020- 039519.
- Boretti A, Banik BK. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition, 2020; 12: 100190. doi: 10.1016/j.phanu.2020.100190.

