Efficacy of Intra-Articular Injection of a Blend of Cross-Linked- Linear Hyaluronic Acid in Knee Osteoarthritis
Barbieri E1, Capparucci I1, Migliore A2, Ferrini F1, Annibalini G1, Carrabs V1,*, Guescini M1, Donati Zeppa S1, Diaferia G3 and Sestili P1
1Dipartimento di Scienze Biomolecolari, Sezione di Scienze motorie e della Salute, University Urbino Carlo Bo, Italy
2Unità di Reumatologia, San Pietro Fatebenefratelli Hospital, Italy
3Dipartimento di Scienza della Vita e Biologia dei Sistemi, Struttura Universitaria di Igiene e Scienze Motorie (SUISM), University of Torino, Italy
Received Date: 31/05/2023; Published Date: 05/10/2023
*Corresponding author: Giosue Annibalini, Dipartimento di Scienze Biomolecolari, Sezione di Scienze motorie e della Salute, University Urbino Carlo Bo, Italy
Abstract
Background: Knee Osteoarthritis (OA) is the most common joint disease in the adult population worldwide: its conservative treatment with intra-articular injections of hyaluronic acid represents one of the most popular options.
Aim: The aim of this study was to retrospectively evaluate the efficacy of intra-articular injections of a preparation containing a blend of Cross-Linked and Linear Hyaluronic Acid (CLHA) in ameliorating joint pain and function in active-OA patients.
Design: A retrospective descriptive study was conducted on subjects who received diagnosis of active knee OA between December 2021 and March 2023. Biochemical - such as cytokine profiling in synovial fluid and urine collagen telopeptide II (CTX-II) levels - and clinical parameters - such as patient-reported values of knee function - have been collected and evaluated.
Setting: Patients were clinically evaluated in Casa di Cura Montanari, Morciano di Romagna (RN), Italy.
Population: Sixty-seven (67) patients, aged 50–65 years, were registered with active knee OA, radiographic Kellgren stage II–III during the observational period. 3 patients were excluded due to violations of inclusion/exclusion criteria, 2 patients refused to be part of the study. Synovial fluid samples (SF) from 40 (64,5%) patients with initial and recurrent effusion after 4 months from baseline, were analyzed for selected cytokines content.
Methods: OA patients were enrolled for study participation after providing written informed consent. Inclusion criteria were the following: Kellgren-Lawrence Grade II or III OA; knee synovial effusion at the diagnosis stage; age range of 35-75 years. Exclusion criteria included: infection in the joint; inflammatory joint disease; osteonecrosis; positive synovial fluid culture; reduced range of motion; large knee circumference (>45 cm); recent intra-articular HA injections and knee trauma or surgery; full-thickness cartilage loss in index knee; use of corticosteroids and other analgesics within the previous 3 months prior to study. Eligible knee OA patients (62) were treated with intra-articular injection(s) of CLHA (cross-linked high molecular weight component of 1-2 million Da plus linear lower molecular weight fraction of 500 KDa). Based on clinical judgment, injections of CLHA were repeated at 4 months (40 patients) and 8 months (29 patients).
Clinical assessment, including visual analogic scale (VAS) for pain, range of motion (ROM) and Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index for knee functional limitation, was performed at baseline and after 4, 8 and 12 months of follow-up. Urine samples were collected and analysed for CTX-II in all timepoints of the study. SF was obtained through arthrocentesis at baseline and at 4 months from patients which showed recurrent intra-articular effusion (40 patients), and was analyzed for IL-1β, IL-6, TNF-α and IL-10 by content by ELLA™ automated immunoassay system.
Results: Intra-articular injections of CLHA invariably improved joint pain and function independently from the age; synovial biochemical analyses indicated the attenuation of inflammatory cytokines concentration and the stabilization of CTX-II.
Conclusions: CLHA represents a highly effective treatment in active knee OA patients.
Keywords: Activated knee osteoarthritis; Hyaluronic acid; Cytokines; CTX-II
Introduction
Knee osteoarthritis (OA) is the most common chronic joint condition and the primary cause of discomfort and limitations in developed countries [1]. Due to a rise in life expectancy, population aging, and the quick development of risk factors like obesity, the incidence of OA is steadily growing [2]. OA symptoms include joint pain, synovitis, a slow loss of articular cartilage, and alterations of the subchondral bone and periarticular tissues [3]. Preventive and pharmacological treatment approaches to reduce inflammation and optimize knee mobility are the cornerstones of OA therapy [4]. Pharmacological therapies used today to treat knee OA focus on symptom relief rather than structural changes. Due to its ability to act on pathologically critical areas and elevated resistance to enzymatic degradation, hyaluronic acid (HA) has been recognized as a safe and successful first-line therapy for OA [5,6]. The effect of HA in OA has often been attributed to its biomechanical and viscoelastic properties, due to its ability to cushion and lubricate the joint [7,8]; however, there is growing evidence suggesting that the benefits of HA also depend on other biological actions on both inflammation and/or cartilage degradation [9–14]. Cross-linked and Linear HA (CLHA) is a commercially available formulation consisting of a mixture of cross-linked high molecular weight HA fraction (MW 1-2 million Da) and a linear lower molecular weight fraction of HA (MW 500 KDa) used as viscosupplement. This formulation makes the polymer more resistant against the degradative action of hyaluronidases; the cross-linked polymer fraction in CLHA is also able to remain longer in the synovial space than the HA linear form.
The aim of the present retrospective case-report study was to evaluate the efficacy of CLHA infiltration in the treatment of active OA pairing clinical and biochemical determinations from a group of eligible cases.
Material and Methods
We performed a retrospective descriptive study with follow-up visits at 4, 8 and 12 months. Patients with symptomatic activated knee OA, grades II–III according to Kellgren–Lawrence (KL) score radiographically examined no longer than three months before the beginning of the study, were enrolled. Inclusion criteria were the following: presence of joint effusion, age range of 35-75 years; index knee Kellgren-Lawrence Grade II or III. Exclusion criteria were the following: joint infection, inflammatory joint disease, osteonecrosis, positive synovial fluid culture, reduced range of motion, large knee circumference (>45 cm), recent HA injections and knee trauma or surgery, full-thickness cartilage loss in index knee and/or treatments with steroids or non-steroidal anti-inflammatory drugs within the previous 3 months, rheumatic pathologies, endocrinopathies, malignancies and systemic diseases.
The study meets the ethical standards of the journal. In particular, all experimental procedures were carried out according to the principles and recommendations described elsewhere [15]. The study was carried out according to the Helsinki Declaration for research with human volunteers and all patients signed an informed consent form to participate. Eligible Knee OA cases were treated at baseline with intra-articular injection of CLHA (Regenflex BioPlus, Regenyal S.r.l., San Benedetto del Tronto, Italy) consisting of a mixture of two fractions, namely a cross-linked high molecular weight HA component sizing 1-2 million Da and a linear lower molecular weight fraction of 500 KDa fraction). Patients (n. 62) received a 3 ml (75 mg/3 mL) injection of Regenflex BioPlus at baseline; additional injections were repeated after 4 (40 patients) or 8 (29 patients) months from baseline based on clinical judgment. No additional injection was required at 12 months. Patients were followed-up for 12 months with visits at baseline (intraarticular injection of study product), and then 4, 8 12 months after the baseline injection. The mixture of the two fractions of HA was spatially ordered in that cross-linked and linear HA were alternatively layered in a “wafer” fashion to obtain peculiar rheological features and a high homogeneity before and after injection. Sixtyseven (67) patients meeting the inclusion criteria were enrolled in the study. Anthropometric data (age, sex, height, weight, and body mass index, body mass index, BMI) were recorded at baseline (Table I). Clinical assessment, including visual analogic scale (VAS) ranging from 0 to 10 for pain [16], range of motion (ROM) was goniometrically-assessed according to [17] and Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index for knee functional limitation. All WOMAC data presented in this report have been normalized using average score on 0 to 10 scales, similarly to other studies [18], which were performed at baseline and after 4, 8 and 12 months. Urine samples were collected and analyzed for collagen telopeptide II (CTX-II LifeSpan, Inc.) in all timepoints of the study. SF was obtained through arthrocentesis at baseline and at 4-months from OA patients which showed chronic intra-articular effusion and IL-1β, IL-6, TNF-α and IL-10 levels were analysed by ELLA™ automated immunoassay system (Bio-techne, Minneapolis, MN, USA). Synovial fluid were loaded in a A16-Plex single-analyte cartridge (1:2 dilution), containing IL-1β/IL-1F2, IL-6 2nd gen, TNF-α 2nd gen and IL-10; ELLA™ was performed as described by Macis et al., 2021 [19].
Results
Sixtyseven (67) patients were selected for the study based on the inclusion criteria. 3 patients were excluded due to violations of inclusion/exclusion criteria, 2 patients refused to be part of the study. The distribution of gender, age, weight, smokers, BMI grade of the sixtytwo (62) cases of the study is described in Table I. Arthrocentesis was performed in all patients at baseline and repeated after 4 months only in cases with synovial effusion recurrence. The total number of 3 ml injections of CLHA administered under ultrasonographic guidance during the 12-month study was 133. Of the 62 patients enrolled who received the first injection, 40 patients received a total of 2 injections (at 0 and 4 months), and 29 patients received a total of 3 injections (at 0, 4 and 8 months) over the 12 months follow-up period. The volume (data not shown) and the occurrence of SF were significantly reduced after 4 months. No dropout and no local/systemic reactions or side effects were noted. As shown in Figure 1A, B, C, the VAS, ROM and WOMAC scores decreased immediately after the first CLHA injection and remained lower over the 12-month follow-up (p<0.05). The CTX-II urinary concentration decreased significantly after 4, 8 and 12 months as compared to baseline values (Figure 1D). Fourty (40, 64,5%) patients suffering from activated knee OA with recurrent effusion were subjected to a second arthrocentesis at 4-months and 4 months-SF cytokine content was compared to that detected in the SF samples collected at baseline. The results are shown in Figure 2, and indicate that the levels of the selected pro-inflammatory markers IL-1β, IL-6 and TNF-α, were significantly reduced after 4 months (p<0.05); notably IL-10 levels, an anti-inflammatory target, only slightly and non-significantly decreased at 4 months (Figure 2).
Table 1: Study group characteristics.
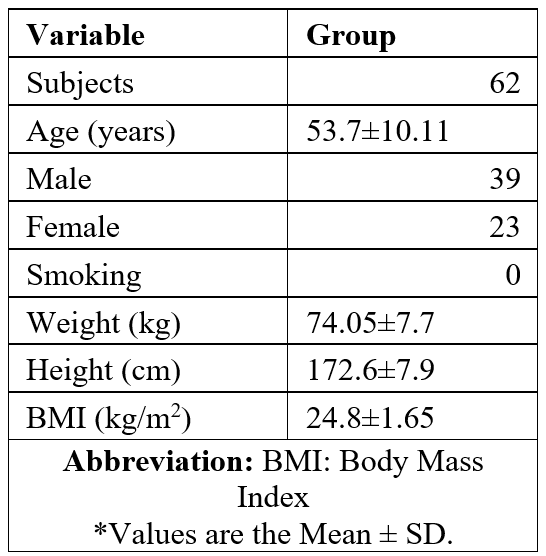
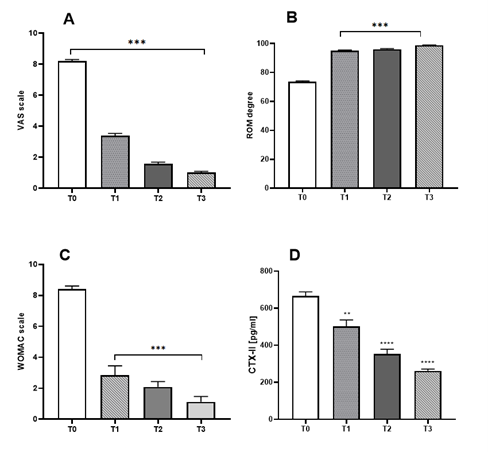
Figure 1: Clinical results at baseline (T0) and after 4 months (T1), 8 months (T2), 12 months (T4) follow-up visits. (A) VAS, Visual analogue scale; (B) ROM, Range of motion (degree); (C) WOMAC, Western Ontario and McMaster Universities Osteoarthritis (functional subscale normalized using average score on 0 to 10 scales); (D) Urinary levels of CTX-II determined at baseline (immediately before the first injection with CLHA). Values are the means ± SEM. *, significantly different from baseline (p<0.05).

Figure 2: Levels of biochemical markers of inflammation in synovial fluids. Synovial IL-1β, IL-6, TNF-α and IL-10 were determined at baseline (immediately before the intra-articular injection of CLHA at baseline) and after 4 months. SF, synovial fluid. Values are means ± SEM. *, significantly different as compared to baseline (p<0.05).
Discussion
Taken together our results suggest that the CLHA formulation used herein promotes a prompt beneficial effect and establishes a favourable and long-lasting condition in the synovial environment. These effects can be commented and tentatively explained taking into account the dual structure - crosslinked and linear - of the polymers in CLHA and the multilayering technique used to mix the two fractions. Indeed, the linear form of HA might promote an immediate relief due to its prompt availability to positively interact with biological targets; moreover, the linear HA layers buffer the excess viscosity typical of crosslinked fractions, that sometimes causes pain following injection due to their rheological friction. Conversely, HA crosslinking reduces HA sensitivity to hyaluronidase-mediated degradation, a property that makes the cross-linked fraction more stable and durable than the linear form. The combination of these activities probably accounts for significant clinical improvements of VAS, ROM and WOMAC scales observed over time. These clinical outcomes are further strengthened by the still positive clinical and functional outcomes at the 12 months follow-up and are consistent with the significant reduction in IL-1β, IL-6 and TNF-α observed at 4 months in the patients with recurrent joint effusion counseled for a second arthrocentesis. Notably, although some of them (n. 29) needed a third injection at 8 months, joint effusion did not develop again in these patients up to the end of the 1-year clinical follow-up.
Our observations are in keeping with the notion that HA, by virtue of its role in joint lubrication and cushioning, may reduce the joint friction coefficient in patients suffering from activated knee OA, which is the main risk factor for degenerative disease and knee pain; secondarily, our data suggest that the rheological properties of HA can be finely tuned and ameliorated by intercalating multiple and alternate layers of linear and crosslinked fractions.
In addition, CLHA, being more resistant to hyaluronidase degradation, confirms its attitude to warrant a prolonged visco-integration and lubricating effect, which, according to the specific program adopted in the present study, could be even longer. Furthermore, we showed that this therapeutic treatment induces significant and lasting improvement in the most severe cases of activated knee OA characterized by recurrent effusion symptoms.
Conclusion
Several forms of HA are available today, yet there is still a need to identify the rational basis for their formulation and combination in a treatment program for activated knee OA. The main features of the treatment herein evaluated, are likely to depend on the specific mode of mixing the two polymer fractions and on the choice of their differential sizes that confer peculiar rheological and biological properties to the preparation.
This interpretation agrees with independent evidence showing that the pharmacodynamic characteristics of HA can be modified according to its size, structure, and chemical variables.
Further studies with a larger sample size will be needed to further evaluate the efficacy profile of Regenflex® BIO-PLUS in patients with OA.
Authorship Criteria: I.C. was responsible for patient recruitment definition and clinical management plan; G.A., M. G., S.D.Z., G.D. and V.C. contributed to the development of the research; E.B., F.F. acquisition of data or analysis and interpretation of data; P.S. was revising the article critically for important intellectual content; A.M. conceptualization and revision.
Conflicts of Interest/ Competing Interests: The authors declare no conflict of interest.
Grant Information: The co-founded doctoral Fellowship of Regione Marche (Progetto di Dottorato Innovativo a caratterizzazione industrial – Cycle XXXV) at the Department of Biomolecular Sciences, University of Urbino Carlo Bo, Urbino, Italy supported Vittoria Carrabs in this research.
This project is partially funded by the research grant "Analysis of biomechanical, functional and bio-molecular responses induced by treatment with hyaluronic acid-based infiltrative medical devices in various clinical areas (rheumatology, orthopedics, traumatology), aimed at the rational development of innovative products" with Regenyal Laboratories Srl of San Benedetto del Tronto (Research contract 19-02-2020 - Authorization from the Biomolecular Sciences Depatment of the University of Urbino n. 316/2019 del 18/12/2019).
The author(s) received no specific funding for this work.
Acknowledgements: Authors thanks Regenyal Laboratories SrL, San Benedetto del Tronto (AP), Italy for providing Regenflex® BIO-PLUS
References
- Ashford S, Williard J. Osteoarthritis. Nurse Pract, 2014; 39: 1–8. doi: 10.1097/01.NPR.0000445886.71205.c4.
- Felson DT. Osteoarthritis: New Insights. Part 1: The Disease and Its Risk Factors. Ann Intern Med 2000; 133: 635. doi:10.7326/0003-4819-133-8-200010170-00016.
- Briggs AM, Woolf AD, Dreinhöfer K, Homb N, Hoy DG, Kopansky-Giles D, et al. Reducing the Global Burden of Musculoskeletal Conditions. Bull World Health Organ, 2018; 96: 366–368, doi:10.2471/BLT.17.204891.
- Primorac D, Molnar V, Matišić V, Hudetz D, Jeleč Ž, Rod E, et al. Comprehensive Review of Knee Osteoarthritis Pharmacological Treatment and the Latest Professional Societies’ Guidelines. Pharmaceuticals, 2021; 14: 205. doi: 10.3390/ph14030205.
- George E. Intra-Articular Hyaluronan Treatment for Osteoarthritis. Ann Rheum Dis, 1998; 57: 637–640. doi: 10.1136/ard.57.11.637.
- Maneiro E, de A.M.F.-S.J.G.F.B.F. The Biological Action of Hyaluronan on Human Osteoartritic Articular Chondrocytes: The Importance of Molecular Weight. Clinical and Experimental Rheumatolgy, 2004.
- Maheu E, Bannuru RR, Herrero-Beaumont G, Allali F, Bard H, Migliore A. Why We Should Definitely Include Intra-Articular Hyaluronic Acid as a Therapeutic Option in the Management of Knee Osteoarthritis: Results of an Extensive Critical Literature Review. Semin Arthritis Rheum, 2019; 48: 563–572, doi: 10.1016/j.semarthrit.2018.06.002.
- Migliore A, B F, G G, S.E.A.M.C.S.C.O.D.L.G.I.C.F. One-Year Follow-up Showing Effects of Single Intra-Articular Injection of Hyaluronic Acid (1,500-2,000 KDa) in Symptomatic Knee Osteoarthritis. Journal of Biological regulator and homeostatic agents.
- Boettger M, Kümmel D, Harrison A, Schaible H-G. Evaluation of Long-Term Antinociceptive Properties of Stabilized Hyaluronic Acid Preparation (NASHA) in an Animal Model of Repetitive Joint Pain. Arthritis Res Ther, 2011; 13: R110. doi:10.1186/ar3394.
- Watterson JR, Esdaile JM. Viscosupplementation: Therapeutic Mechanisms and Clinical Potential in Osteoarthritis of the Knee. Journal of the American Academy of Orthopaedic Surgeons, 2000; 8: 277–284. doi:10.5435/00124635-200009000-00001.
- Brun P, Panfilo S, Daga Gordini D, Cortivo R, Abatangelo G. The Effect of Hyaluronan on CD44-Mediated Survival of Normal and Hydroxyl Radical-Damaged Chondrocytes. Osteoarthritis Cartilage, 2003; 11: 208–216. doi: 10.1016/S1063-4584(02)00352-7.
- Brun P, Zavan B, Vindigni V, Schiavinato A, Pozzuoli A, Iacobellis C, et al. In Vitro Response of Osteoarthritic Chondrocytes and Fibroblast-like Synoviocytes to a 500-730 KDa Hyaluronan Amide Derivative. J Biomed Mater Res B Appl Biomater, 2012; 100B: 2073–2081. doi:10.1002/jbm.b.32771.
- Julovi SM, Yasuda T, Shimizu M, Hiramitsu T, Nakamura T. Inhibition of Interleukin-1? Stimulated Production of Matrix Metalloproteinases by Hyaluronan via CD44 in Human Articular Cartilage. Arthritis Rheum, 2004; 50: 516–525. doi: 10.1002/art.20004.
- Karna E, Miltyk W, Surażyński A, Pałka JA. Protective Effect of Hyaluronic Acid on Interleukin-1-Induced Deregulation of Β1-Integrin and Insulin-like Growth Factor-I Receptor Signaling and Collagen Biosynthesis in Cultured Human Chondrocytes. Mol Cell Biochem, 2008; 308: 57–64. doi:10.1007/s11010-007-9612-5.
- Barbieri E, Capparucci I, Mannello F, Annibalini G, Contarelli S, Vallorani L, et al. Efficacy of a Treatment for Gonarthrosis Based on the Sequential Intra-Articular Injection of Linear and Cross-Linked Hyaluronic Acids. Muscle Ligaments and Tendons Journal, 2019; 09: 606. doi:10.32098/mltj.04.2019.17.
- Hawker GA, Mian S, Kendzerska T, French M. Measures of Adult Pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF. Arthritis Care Res (Hoboken), 2011; 63: S240–S252, doi:10.1002/acr.20543.
- Lenssen AF, van Dam EM, Crijns YH, Verhey M, Geesink RJ, van den Brandt PA, et al. Reproducibility of Goniometric Measurement of the Knee in the In-Hospital Phase Following Total Knee Arthroplasty. BMC Musculoskelet Disord, 2007; 8: 83. doi:10.1186/1471-2474-8-83.
- Bellamy N, Wilson C, Hendrikz J. Population-Based Normative Values for the Western Ontario and McMaster (WOMAC) Osteoarthritis Index: Part I. Semin Arthritis Rheum, 2011; 41: 139–148. doi: 10.1016/j.semarthrit.2011.03.002.
- Macis D, Aristarco V, Johansson H, Guerrieri-Gonzaga A, Raimondi S, Lazzeroni M, et al. A Novel Automated Immunoassay Platform to Evaluate the Association of Adiponectin and Leptin Levels with Breast Cancer Risk. Cancers (Basel), 2021; 13: 3303. doi:10.3390/cancers13133303.

