A Real-World User Survey on the Effectiveness of a Hypertonic Seawater Nasal Spray as an Add-On to Pharmacological Treatment in Patients with ENT Diseases
Stella Georgiou and Konstantinos Alevizopoulos*
Research & Development Department, Gerolymatos International S.A, Greece
Received Date: 21/02/2023; Published Date: 17/04/2023
*Corresponding author: Konstantinos Alevizopoulos, PhD, Research & Development Department, Gerolymatos International S.A, 13 Asklipiou Str. 14568, Kryoneri, Attica, Greece
Abstract
A real-world, user survey study was conducted in 60 patients who visited pharmacies with a prescription to use nasal corticosteroids or vasoconstrictors for an underlying ENT condition. Patients were offered a hypertonic seawater solution (HSS-Mini) as an add-on treatment for a period of up to two weeks. At the end of the evaluation period, the product’s efficacy, its use pattern, and symptom severity before and after treatment were scored in questionnaires. Users were highly satisfied with the nasal spray; 93.6% was satisfied with the product, 91.6% with its efficacy, and 93.3% with the overall efficacy from its combined use with medication. Reduction of medicated product intake was reported by 93.4% of users. Users were willing to use HSS-Mini independently of medication and recommend it to other users. Overall, a significant reduction of all sinonasal symptoms including stuffy/blocked nose, runny nose, sneezing, itchy/dry nose, or other nasal symptoms was observed (P<0.0001). Quality of life symptoms such as fatigue, reduced productivity, sleep quality, emotional tiredness, and overall feeling were also improved (P<0.0001). Combined with high user satisfaction and willingness for future use, the study results support adjunct use of HSS-Mini for optimal symptom management in sinonasal diseases.
Keywords: Nasal spray; User survey; Hypertonic seawater solutions; Sinonasal symptoms; Nasal congestion; Quality of Life symptoms
Introduction
Individuals suffering from sinonasal diseases experience discomfort due to nasal and sinus symptoms adversely affecting their quality of life. Although the standard care in the treatment of ENT diseases includes antihistamines, nasal corticosteroids, vasoconstrictors, and other prescription agents [1], troubled individuals often employ nasal irrigation either as a standalone or as adjunct to medicated treatment for optimal symptom relief [2-4].
Overall, both isotonic (0.9% NaCl) or hypertonic (>0.9% NaCl) solutions are used as a means to cleanse the nasal cavity by mechanically removing crusts, mucus, bacteria/viruses, and inflammatory mediators [5]. Hypertonic solutions offer additional benefits due to osmotic effects and are generally considered superior in action to isotonic solutions [5-6]. Among the different solutions used, hypertonic seawater solutions of 2.3% NaCl are the best characterized so far in clinical trials in adult and/or pediatric populations collectively offering effective non-pharmacological symptom relief in patients with sinonasal disorders [7-14].
Although nasal irrigation has been advocated for its effectiveness, scarce data exist regarding user experiences when practicing it in real life. In addition, there is limited knowledge on consumer perceptions with regards to medical devices for nasal rinsing, and whether such treatments are effective and/or user-friendly. In this user survey, we sought to explore user satisfaction and clinical efficacy of HSS-Mini, a hypertonic seawater nasal spray (2.3% NaCl) regarding sinonasal and quality-of-life symptom improvement in patients suffering from ENT diseases.
Methods
Study setting, patients, and medical device used
A prospective user survey study was conducted in 60 patients visiting three pharmacies in Slovenia with a physician’s prescription to use nasal corticosteroids or vasoconstrictors for an underlying ENT condition. HSS-Mini, a hypertonic seawater solution of 2.3% NaCl (Sinomarin® Mini) approved as a medical device for nasal decongestion and cleansing was offered to the patients as an add-on to their prescribed medication, for continuous use of up to 14 days as per the product’s instructions for use. The users were instructed to spray the product 5 minutes before the medicinal product or use it anytime during the day independently of the use of medication. All patients received written questionnaires and instructions on how to fill and return them at the end of the evaluation period.
Study questionnaire
The questionnaire included 10 questions evaluating the product’s efficacy, use pattern, sinonasal, and life-quality symptom severity before and after HSS-Mini treatment. Patient age, product evaluation, and user satisfaction were also recorded (Figure 1). Likert scales were used for scoring sinonasal and life-quality symptoms, the timing and/or the frequency of nasal spray use, the type of the prescribed product used, the consumer’s experience, and their attitude towards future purchase of the product.
Statistical Analysis
Summaries were based on mean + standard deviation (SD) before and after treatment scoring. The distributional properties of change of scoring after treatment were assessed by paired samples t-test. The magnitude of change was expressed by the mean change followed by the corresponding 95% confidence interval. Data were analyzed in SPSS v21 software. All tests were 2-sided and the level of statistical significance was set at α=5%.
Results
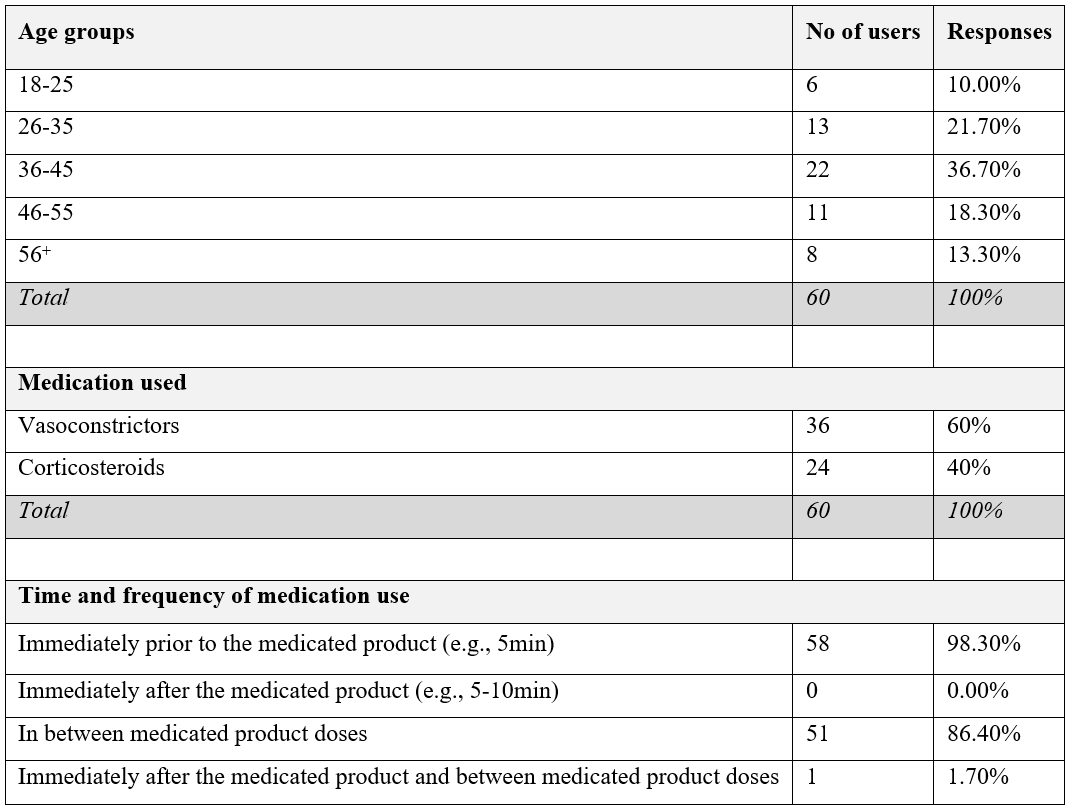
Sixty patients participated in this study. The most represented age group (36.7%) was 36-45 years old. 60% of the participants were prescribed vasoconstrictors; 40.0% used corticosteroids. 98.3% used HSS-Mini 5 minutes before medication use (as instructed); 86.4% of the users in between medication dose schemes; 1.7% both after and between medication doses (Table 1).
Table 1: Age groups, medication types and nasal spray use patterns.
Overall efficacy, satisfaction and evaluation
93.3% of the users were satisfied with the use of HSS-Mini and 91.6% with its overall efficacy. 93.3% expressed satisfaction from the overall efficacy of the combined treatment (Table 2).
Table 2: Consumer satisfaction and product efficacy.

93.4% stated that “HSS-Mini allowed them to reduce the overall medicated product intake”. 98.4% would further endorse its use while 90.0% “would consider using HSS-Mini alone, without medication”. 83.3% reported that they would purchase the product in the future (Table 3).
Table 3: Evaluation of HSS-Mini and consumer attitudes.

Sinonasal and life-quality symptoms
Following spraying with HSS-Mini and medication, the users responded as “moderately troubled” to “not troubled” in nasal congestion, runny nose, sneezing, itchy/dry nose, or other problems (Figure 2). Regarding life-quality symptoms, the users responded “not troubled” in reduced productivity, poor sleep quality and emotional tiredness, and “hardly troubled at all” in fatigue. The distribution of symptoms was also reduced in all symptoms assessed (Figure 3). 100.0% of participants reported reduced runny nose (P<0.0001), 98.3% less congestion (P<0.0001), 91.5% less sneezing (P<0.0001), and 91.7% less itchy/dry nose (P<0.0001). Other sinonasal symptoms were also reduced in 93.3% of the users (P<0.0001) (Figure 4, Table 4). Similarly, for life-quality symptoms, after HSS-Mini use, 96.7% of users felt less fatigue (P<0.0001), 85.0% less emotional tiredness (P<0.0001), 98.3% less disturbed sleep (P<0.0001), and 93.3% less compromised productivity (P<0.0001). The overall feeling score was also reduced (P<0.0001) in 93.3% of the total population (Figure 5, Table 4). As with nasal symptoms, all life-quality symptoms improved following treatment.
Table 4: Symptom score reduction.
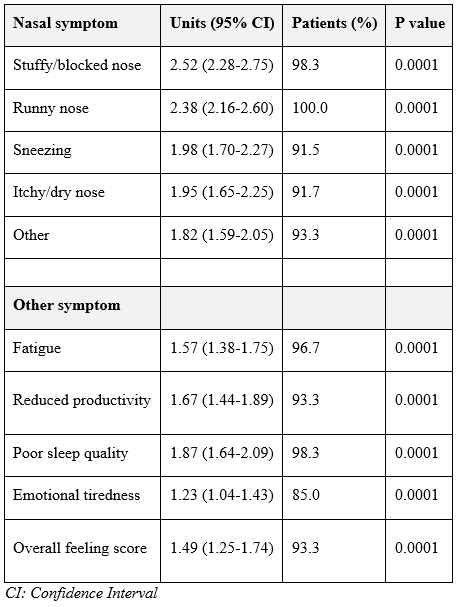


Figure 1: HSS-Mini user survey questionnaire.

Figure 2: Distribution of sinonasal symptoms before and after nasal spraying with HSS-Mini and medication use. Other problems: eyes, throat, etc.
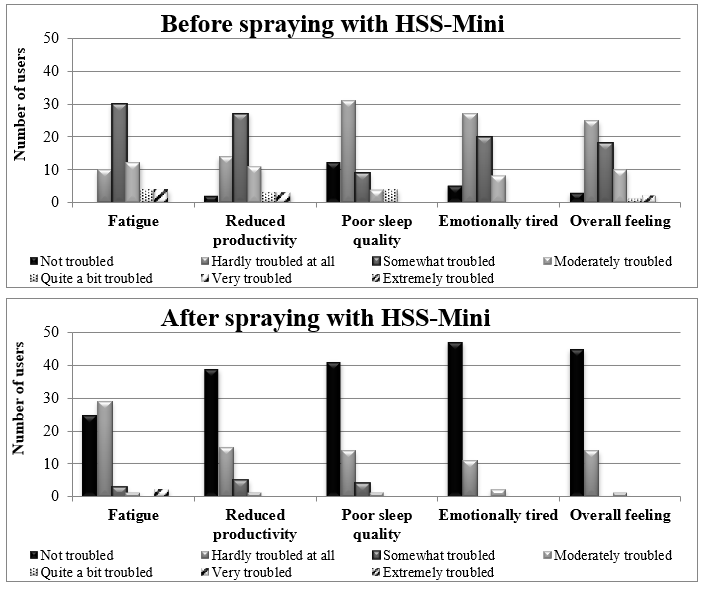
Figure 3: Distribution of life-quality symptoms before and after nasal spraying with HSS-Mini and medication use.
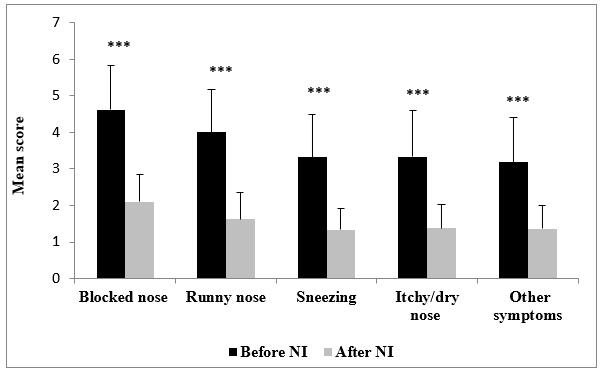
Figure 4: Paired nasal symptom mean scores before and after nasal spraying (NI) with HSS-Mini and medication use (NI). *** P<0.0001
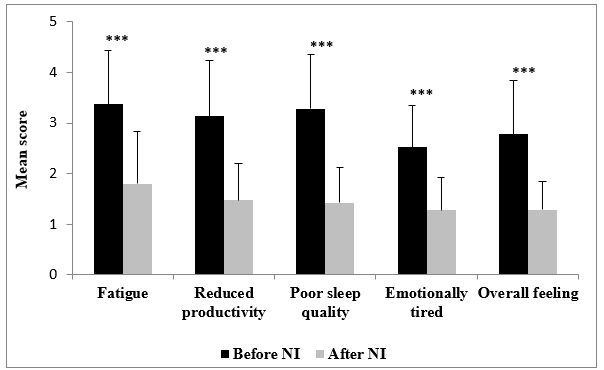
Figure 5: Paired mean score of symptoms related to patient quality of life before and after nasal spraying (NI) with HSS-Mini and medication use (NI). *** P<0.0001
Discussion
Nasal rinsing is a well-established practice for managing symptoms during ENT diseases [1-4]. Both isotonic (0.9% NaCl) and hypertonic (>0.9% NaCl) irrigation solutions are used in daily practice [5]. In this real-world user survey study, we aimed to source information on HSS-Mini’s efficacy and its use pattern, and to evaluate sinonasal and life-quality symptoms in patients with ENT disorders utilizing the product as an add-on to prescribed pharmacological therapy.
Our results showed high user satisfaction. Briefly, the majority of users were very satisfied with the product itself, and with its efficacy upon use with or without medication. Importantly, a reduction in overall medicated product intake was observed. Very high user satisfaction is reflected in their recommendation of the product to counterparts and eagerness for future purchase. The majority of users would even consider using the nasal spray alone without medication. This is an important study result indicating that the nasal spray offered effective symptom control and allowed reduced use of medication; this property is frequently sought by consumers.
Analyzing sinonasal symptoms before and after treatment, use of HSS-Mini with medication contributed to a significant reduction of sinonasal symptom burden including blocked/runny nose, sneezing, and itchy/dry nose. Similarly, life-quality symptoms such as fatigue, reduced productivity, sleep quality, and overall feeling were improved. These results are in agreement with previously published clinical results with the same 2.3% NaCl hypertonic solution showing excellent tolerability and efficacy in adult and pediatric rhinitis patients when the solution was used either as a standalone treatment [7,15] or in combination with medication [8].
Conclusion
HSS-Mini is a product providing high user satisfaction and efficacy in managing ENT symptoms and improving patient quality of life. Our results support continuous adjunct use of this nasal device together with prescribed medication in sinonasal disorders.
Author contributions: Conceptualization and methodology: KA; Data processing and analysis: SG, KA; Literature review: SG, KA; Manuscript preparation: SG, KA.
Conflicts of Interest/ Competing Interests: No conflict of interest was declared by the authors.
Grant Information: The author(s) received no specific funding for this work.
Competing interest and grant information: I confirm that there is no competing interest to declare and I did not receive any grant to conduct this study.
Acknowledgements: We would like to thank Miss Claire Challoner, Dr. Nazoorah Malek, Dr. Ala Abdullah, Dr. Neelam Agrawal and Dr Sheena Johns in helping us to collect the data.
References
- Chong LY, Head K, Hopkins C, Philpott C, Glew S, Scadding G, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database Syst Rev, 2016, 4(4): CD011995. doi: 10.1002/14651858.CD011995.pub2. PMID: 27115216; PMCID: PMC8078614.
- King D, Mitchell B, Williams CP, Spurling GK. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst Rev, 2015; 2015(4): CD006821. doi: 10.1002/14651858.CD006821.pub3. PMID: 25892369; PMCID: PMC9475221.
- Head K, Snidvongs K, Glew S, Scadding G, Schilder AG, Philpott C, Hopkins C. Saline irrigation for allergic rhinitis. Cochrane Database Syst Rev, 2018; 6(6): CD012597. doi: 10.1002/14651858.CD012597.pub2. PMID: 29932206; PMCID: PMC6513421.
- Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology, 2020; 58(Suppl S29): 1-464. doi: 10.4193/Rhin20.600. PMID: 32077450.
- Principi N, Esposito S. Nasal Irrigation: An Imprecisely Defined Medical Procedure. Int J Environ Res Public Health. 2017; 14(5): 516. doi: 10.3390/ijerph14050516. PMID: 28492494; PMCID: PMC5451967.
- Kanjanawasee D, Seresirikachorn K, Chitsuthipakorn W, Snidvongs K. Hypertonic Saline Versus Isotonic Saline Nasal Irrigation: Systematic Review and Meta-analysis. Am J Rhinol Allergy, 2018; 32(4): 269-279. doi: 10.1177/1945892418773566. Epub 2018 May 18. PMID: 29774747.
- Freche C, Castillo L, De Corbiere S, Dessi P, Fontanel JP, Jankowski R, et al. Usefulness of hypertonic seawater (Sinomarin®) in rhinology; Rev Officiel de la Societ Franc d’ ORL, 1998; 50: 73-75.
- Gonzalez G, Sanchez AY, Mejia R. An investigational, prospective, longitudinal, comparative, multicentre, open-label study on the efficacy and tolerability of Sinomarin Spray for the treatment of rhinitis. J Fed Otolaryngol Col Soc Mexican Republic, 2008.
- Lee SH, Jong JS, Lee SH, Hwang SJ, Lee HM. Effect of Hypertonic Seawater (Sinomarin®) on Mucociliary Clearance in Normal Subjects. J Rhinol, 2003; 10(1,2): 19-22.
- Süslü N, Bajin MD, Süslü AE, Oğretmenoğlu O. Effects of buffered 2.3%, buffered 0.9%, and non-buffered 0.9% irrigation solutions on nasal mucosa after septoplasty. Eur Arch Otorhinolaryngol, 2009; 266(5): 685-689. doi: 10.1007/s00405-008-0807-5. Epub 2008 Sep 18. PMID: 18802718.
- Bencova A, Vidan J, Rozborilova E, Kocan I. The impact of hypertonic saline inhalation on mucociliary clearance and nasal nitric oxide. J Physiol Pharmacol, 2012; 63(3): 309-313. PMID: 22791646.
- Köksal T, Çizmeci MN, Bozkaya D, Kanburoğlu MK, Şahin Ş, Taş T, et al. Comparison between the use of saline and seawater for nasal obstruction in children under 2 years of age with acute upper respiratory infection. Turk J Med Sci, 2016; 46(4): 1004-1013. doi: 10.3906/sag-1507-18. PMID: 27513397.
- Kurtaran H, Ugur KS, Yilmaz CS, Kaya M, Yuksel A, Ark N, et al. The effect of different nasal irrigation solutions following septoplasty and concha radiofrequency: a prospective randomized study. Braz J Otorhinolaryngol. 2018; 84(2): 185-190. doi: 10.1016/j.bjorl.2017.01.005. Epub 2017 Feb 22. PMID: 28325622; PMCID: PMC9449243.
- Perić A, Kovačević SV, Barać A, Gaćeša D, Perić AV, Jožin SM. Efficacy of hypertonic (2.3%) sea water in patients with aspirin-induced chronic rhinosinusitis following endoscopic sinus surgery. Acta Otolaryngol, 2019; 139(6): 529-535. doi: 10.1080/00016489.2019.1605454. Epub 2019 Apr 29. PMID: 31035841.
- Liu Z, Wang H. [The clinical research of 2.3% hypertonic salt water in the treatment of patients with chronic rhinitis]. In J Taishan Med College, 2015; 35(7).

