Sars-Cov-2 Placentitis Complicating Maternal Covid-19 Infection
Calista J Lambert1, Fiona Chan2 and Julia Unterscheider1,3,*
1Department of Maternal Fetal Medicine, The Royal Women’s Hospital, Australia
2Department of Anatomical Pathology, The Royal Women’s Hospital, a division of The Royal Children’s Hospital Laboratory Services, Australia
3Department of Obstetrics and Gynaecology, The University of Melbourne, Australia
Received Date: 08/12/2022; Published Date: 30/12/2022
*Corresponding author: Julia Unterscheider, MD PhD FRANZCOG, Department of Maternal Fetal Medicine, The Royal Women’s Hospital, Melbourne, Victoria, Australia. Department of Obstetrics and Gynaecology, The University of Melbourne, 20 Flemington Rd, Parkville VIC 3052, Melbourne, Australia
Abstract
SARS-CoV-2 infection in pregnancy correlates to a characteristic pattern of placental histopathological changes described as COVID placentitis and may lead to adverse perinatal outcomes including preterm birth and stillbirth. This case series describes 5 cases of COVID placentitis identified on placental histopathology and its associated antenatal course and perinatal outcome. SARS-CoV-2 infection occurred at a mean gestation of 31 weeks and a median of 14 days prior to birth. All cases were observed during the delta wave in a single quaternary maternity centre in metropolitan Melbourne, one of the most locked down cities in the world in 2021. The women suffered mild (n=3), moderate (n=1) or severe disease requiring admission to the intensive care unit (n=1). One woman was double vaccinated. There were two fetal deaths in utero observed (FDIU) at 28 and 36 weeks respectively. Two women delivered preterm (27+2 and 33+5 weeks). Interestingly, the woman with the most severe symptoms was delivered of a healthy, term neonate. The disconnect between maternal symptom severity and fetal outcome is similar to previously reported cases. We acknowledge the selection bias of this small case series.
Keywords: COVID; SARS-CoV-2; Placentitis; Perivillous fibrin deposition; Histiocytic intervillousitis; Trophoblast necrosis
Introduction
Pregnancy is a risk factor for severe COVID and associated with higher rates of maternal and neonatal admissions to ICU, maternal death, preterm birth, and stillbirth [1-3]. Large studies in vaccinated pregnant women with COVID are unavailable due to the recency of population wide vaccination and anecdotally reduced rates of adverse outcomes with recent variants.
SARS-CoV-2 infection has been associated with an emerging pattern of histiocytic intervillositis, perivillous fibrin deposition and/or trophoblast necrosis collectively known as COVID placentitis [4-8] (Figures 1-3). Placental SARS-CoV-2 virus is often isolated, but not required for diagnosis. Recent studies suggest that for mothers infected with SARS-CoV-2, COVID placentitis affects up to 1.5% of births overall with a higher incidence in preterm births (6%) [9,10]. COVID placentitis is associated with significant fetal mortality and morbidity with case reports suggesting high rates of stillbirth (42-49%), preterm births (70%) and low 5-minute APGAR scores (35%) [5,9].
In this series, cases were diagnosed between August and December 2021 when the dominant variant was the virulent and severe delta variant [11]. Prior to July 2021, maternal COVID was rare in Australia due to public health measures. Vaccination in pregnancy was endorsed by professional bodies from June 2021 with a 2-vaccine course considered full vaccination. This study aimed to describe the outcomes of pregnancies with identification of COVID placentitis on placental histopathology at one centre, the Royal Women’s Hospital, a quaternary maternity hospital in Melbourne.

Figure 1: Perivillous fibrin deposition.
Macroscopic appearance of placenta affected by COVID placentitis following fixation in formalin. Patchy pale areas indicate massive perivillous fibrin deposition, occupying over 50% of the placental parenchyma.
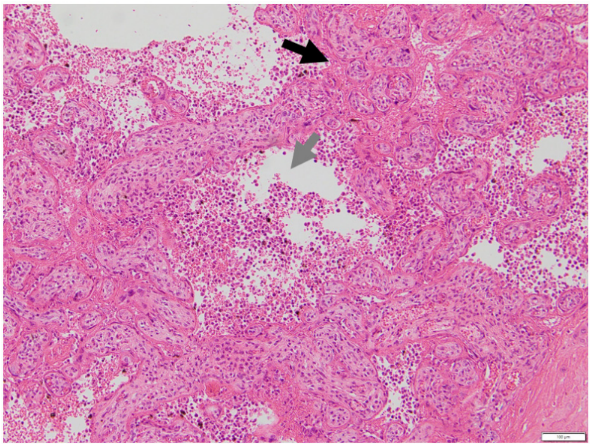
Figure 2: Histiocytic intervillositis.
Placental section with haematoxylin and eosin stain at x100 magnification. The black arrow shows chorionic villi that are adherent and clumped together with of obliteration of the intervillous space. The grey arrow shows massive chronic histiocytic intervillositis. For comparison, images of normal placental villi have been previously published [8].
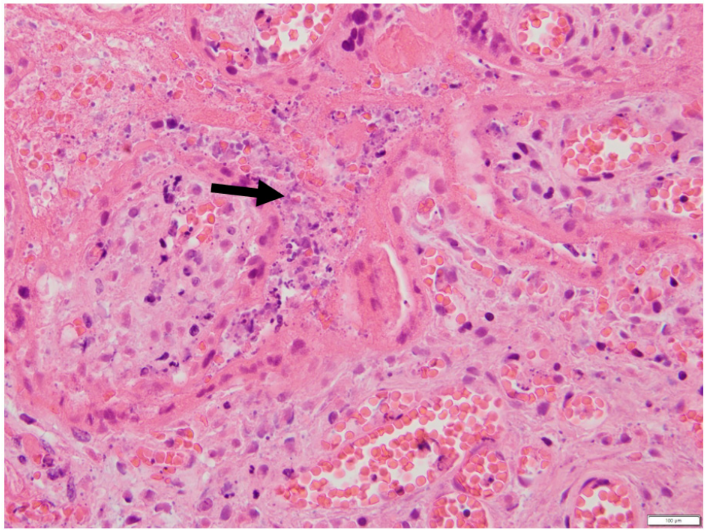
Figure 3: Trophoblast necrosis and perivillous fibrin deposition.
Placental section with haematoxylin and eosin stain at x400 magnification demonstrating prominent syncytiotrophoblast necrosis (black arrow).
Case Series
Five women (mean age 28.8, range 20-39) were diagnosed with SARS-CoV-2 infection at a mean gestational age of 31 weeks (range 25+2 weeks – 36+0 weeks) (Table 1). None of the women were overweight, smoked or had medical comorbidities, all had uncomplicated pregnancies prior to infection and were from diverse ethnicities (n=1 for African, Arab, central Asian, south Asian, and Jewish backgrounds). One woman was fully vaccinated >14 days prior to SARS-CoV-2 infection. The remainder were either unvaccinated (n=3) or partially vaccinated (n=1).
Three women had mild COVID disease, one moderate disease requiring overnight admission and one severe disease requiring ICU admission, remdesivir, tocilizumab, dexamethasone, and intubation for type 1 respiratory failure. Four women received antenatal care and were delivered 13-15 days following SARS-CoV-2 infection; the remaining woman tested positive on the day of delivery. The women with moderate and severe disease had caesarean sections, one for fetal bradycardia and one for deteriorating maternal condition at term. The women with mild disease included one spontaneous preterm vaginal birth of dichorionic-diamniotic (DCDA) twins and two preterm male FDIUs.
There were two female and two male live neonates born with 5-minute APGARs of 7 or above. No antenatal corticosteroids were administed and no cord gases were available. The twins had normal serial sonographic surveillance prior to presentation. One male DCDA twin and one female neonate had birth weights <1st centile for gestational age.
There were two FDIUs in this cohort. FDIU #1 occurred at an estimated gestational age of 27 weeks in a recent refugee without antenatal care. The infant weighed 1010g with external examination suggestive of mild dysmorphic features. Full post-mortem examination was declined; however, a chromosomal microarray returned a normal result. FDIU #2 occurred at 36 weeks’ gestation following presentation for decreased fetal movements. The infant weighed 2462g and further post-mortem examination was declined.
The female neonate born at 27+2 weeks via emergency caesarean for fetal bradycardia following presentation with decreased fetal movements was diagnosed with bilateral intraventricular haemorrhage (left grade IV, right grade II) and bilateral stage 1 retinopathy of prematurity. The twins born at 33+5 weeks’ gestation did not develop long term complications of prematurity. Interestingly, the woman with the most severe COVID disease had a healthy, term neonate.
The placentae weighed between 178-522g with most on the 10-50th centile for gestational age (n=4, remaining placenta on 3rd-5th centile). Perivillous fibrin deposition (n=5), histiocytic intervillositis (n=4), syncytiotrophoblast necrosis (n=4) and SARS-CoV-2 virus (n=2, rest not tested) were identified on placental histopathology. E.coli was cultured from the twin placenta. Additionally, the FDIU had massive perivillous fibrin deposits and features of placental infarction (n=2) and cultured K. oxytoca (n=1).
Table 1: Detailed case descriptions.

Table key: F = female, G = gravidity, P = parity, D1 = Day 1, EmCS = emergency caesarean section. BW: Birth weight in grams (birth weight centile)
Discussion
Studies investigating COVID placentitis have, to date, consisted of basic science or case reports with most coming from 2 large case series of 60 and 61 cases respectively [5,9]. COVID placentitis is considered an uncommon but serious complication of COVID in pregnancy [5,9].
80% (n=4) of the women affected by COVID placentitis in this series were partially or unvaccinated and were diagnosed during the period when the rate of fully vaccinated pregnant women rose from 69% to 85% at our institution. Both stillbirths occurred in unvaccinated women. Vaccination against COVID-19 is possibly protective against COVID placentitis, however this association is made with caution. Interestingly, at the Royal Women’s Hospital no cases of COVID placentitis have been diagnosed since January 2022, coinciding with high rates of vaccinated women and the Omicron variant in Australia.
These cases did not show a correlation between severity of maternal disease and fetal outcome, a finding consistent with the literature [5,9]. The most unwell woman had a healthy neonate and both FDIU occurred in women with mild disease. The interval from infection to delivery was 13-15 days in this series. Previous cases have reported the shorter median intervals from infection to delivery at 4.5 days overall and 10 days for stillbirth (range 0-51 days) [9]. The incidence of growth restricted neonates is higher than previously reported at 33% with 1st centile birth weights (n=2), although this is confounded by the inclusion of one DCDA twin. The literature suggests that COVID placentitis is associated with a lower rate of growth restriction (5-8%), hypothesised to be due the severity of COVID placentitis resulting in a short interval from development to birth [5,9]. Notably, these cases were in cohorts that were older (median age 32 vs. 28.8), more overweight (71% with a BMI >25 vs. 0%) and with more comorbidities (22% with a cardiovascular comorbidity vs. 0%) than this series. It is possible that the health of the mothers in this series facilitated a longer interval from infection to delivery, in turn allowing time for the development of growth restriction, although this hypothesis is made with caution.
40% (n=2) of the women in this series delivered preterm and 40% (n=2) had stillbirths. Despite demographic differences between the women included in this case series and those in the literature, rates of preterm and stillbirth remain similar (stillbirth rate 42-49%; preterm birth rate 70%) and are significantly higher than COVID positive women overall (stillbirth rate 1%; preterm birth rate 14%) [1,5,9]. The variance in phenotypes of COVID placentitis make clinical management difficult as placental phenotype seems related to non-modifiable maternal characteristics and unrelated to factors amenable to antenatal diagnosis or surveillance. Vaccination may decrease but not completely mitigate risk as the sole fully vaccinated woman in this series was delivered extremely preterm for fetal distress with a relatively severe phenotype of COVID placentitis. These findings raise difficult choices for maternity clinicians about for whom and when to intervene as whilst COVID placentitis itself is an uncommon complication of COVID, serious complications are common [1,5,9].
The pattern of perivillous fibrin deposition, histiocytic intervillositis and/or trophoblast necrosis can range from being localised to small areas of the placental parenchyma in well neonates to exceeding 90% of the placenta in FDIU, leading to the hypothesis that the severity of COVID placentitis causes FDIU due to placental dysfunction [4,9]. The findings of this series support the hypothesis as both FDIU had diffuse or massive perivillous fibrin deposits whist live neonates were described as having ‘increased, prominent, multifocal or localised’ histiocytic intervillositis and perivillous fibrin deposition [4, 9]. SARS-CoV-2 virus was identified in the two placentae that tested for its presence, consistent with the literature [4,5,8,9,12]. To date, there is insufficient evidence in the literature to explain the pathophysiology of COVID placentitis or any links between COVID placentitis and infection with different variants of SARS-CoV-2 virus.
To our knowledge, this case series is the first to report vaccination status and to include a fully vaccinated woman, likely due to the relative recency of widespread vaccination. Few cases have been previously reported, so the congruence of findings with these cases strengthens provisional hypotheses.
Weaknesses include the small number of cases (n=5) and the retrospective, non-systematic method by which cases were identified. Cases were identified based on placental histopathological findings consistent with COVID placentitis and retrospectively reviewed with data drawn from the electronic medical records system. Placental histopathology was not systematically performed on all COVID-positive women delivered at this hospital.
Conclusion
COVID placentitis is a histopathological diagnosis based on a pattern of perivillous fibrin deposition, histiocytic intervillositis and trophoblast necrosis. Although an uncommon complication of maternal SARS-CoV-2 infection, COVID placentitis carries a high rate of stillbirth (40-49%), preterm birth (40-70%), no clinically predictive factors and may affect any SARS-CoV-2 positive pregnant woman. Its incidence may be reduced with vaccination however this hypothesis is made with caution.
Author Contributions: CL, FC and JU developed the concept of the case series. FC oversaw the analysis of placental histopathology. CL extracted data from the electronic medical record, analysed the data and wrote the manuscript. FC and JU edited the manuscript. All authors approved the final manuscript.
Acknowledgements:
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research was not financially supported.
References
- Vousden N, Bunch K, Morris E, Simpson N, Gale C, O’Brien P, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: A national cohort study using the UK Obstetric Surveillance System (UKOSS). PLOS One, 2021; 16(5): e0251123.
- Knight M, Bunch K, Tuffnell D, Patel R, Shakespeare J, Kotnis R, et al. Saving Lives, Improving Mother’s Care - Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2017-19. Oxford: Oxford University; 2021.
- Thoma ME, Declercq ER. All-Cause Maternal Mortality in the US Before vs During the COVID-19 Pandemic. JAMA Netw Open, 2022; 5(6): e2219133.
- Fitzgerald B, O'Donoghue K, McEntagart N, Gillan JE, Kelehan P, O'Leary J, et al. Fetal Deaths in Ireland Due to SARS-CoV-2 Placentitis Caused by SARS-CoV-2 Alpha. Arch Path Lab Med, 2022; 146(5): 529-537.
- Stenton S, McPartland J, Shukla R, Turner K, Marton T, Hargitai B, et al. SARS-COV2 placentitis and pregnancy outcome: A multicentre experience during the Alpha and early Delta waves of coronavirus pandemic in England. eClinicalMedicine, 2022; 47: 101389.
- Garrido-Pontnou M, Navarro A, Camacho J, Crispi F, Alguacil-Guillén M, Moreno-Baró A, et al. Diffuse trophoblast damage is the hallmark of SARS-CoV-2-associated fetal demise. Mod Pathol, 2021; 34(9): 1704-1709.
- Schwartz DA, Bugatti M, Santoro A, Facchetti F. Molecular Pathology Demonstration of SARS-CoV-2 in Cytotrophoblast from Placental Tissue with Chronic Histiocytic Intervillositis, Trophoblast Necrosis and COVID-19. Journal of Developmental Biology, 2021; 9(3): 33.
- Linehan L, O'Donoghue K, Dineen S, White J, Higgins JR, Fitzgerald B. SARS-CoV-2 placentitis: An uncommon complication of maternal COVID-19. Placenta, 2021; 104: 261-266.
- Redline RW, Ravishankar S, Bagby C, Saab S, Zarei S. Diffuse and Localized SARS-CoV-2 Placentitis: Prevalence and Pathogenesis of an Uncommon Complication of COVID-19 Infection During Pregnancy. Am J Surg Pathol, 2022; 46(8): 1036-1047.
- Thomas J, Sun Y, Debelenko L. Infrequent Placental and Fetal Involvement in SARS-CoV-2 Infection: Pathology Data from a Large Medical Center. J Dev Biol, 2021; 9(4): 45.
- Wrenn JO, Pakala SB, Vestal G, Shilts MH, Brown HM, Bowen SM, et al. COVID-19 severity from Omicron and Delta SARS-CoV-2 variants. Influenza Other Respir Viruses, 2022; 16(5): 832-836.
- Mao Q, Chu S, Shapiro S, Young L, Russo M, De Paepe ME. Placental SARS-CoV-2 distribution correlates with level of tissue oxygenation in COVID-19-associated necrotizing histiocytic intervillositis/perivillous fibrin deposition. Placenta, 2022; 117: 187-193.

