Three Cases of Under Diaphragmatic Digestive Duplication
Rajaonarison Ny Ony Narindra LH1,*, Tombomiadantsoa Biendoux2, Rafaralahivoavy TR3, Ahmad Ahmad1 and Hunald Francis Allen2
1Medical Imaging Center, CHU HJRA Antananarivo, Madagascar
2Paediatric Surgery Department, CHU JRA Antananarivo, Madagascar
3Medical Imaging Department, CHU Andrainjato Fianarantsoa, Madagascar
Received Date: 28/06/2022; Published Date: 06/07/2022
*Corresponding author: Rajaonarison Ny Ony Narindra Lova Hasina, Medical Imaging Center, CHU HJRA Antananarivo, Madagascar
Abstract
Digestive duplication, communicating or not, is a rare congenital malformation characterized by the presence of a fluid pocket at the expense of the wall of a digestive tract. Frequently spherical than tubular, the sub-diaphragmatic location is characterized by the presence of an abdominal mass with digestive disturbances and recurrent abdominal pain. We report three cases of under diaphragmatic digestive duplication, revealed by medical imaging and confirmed by pathology.
Keywords: Digestive duplication; Abdominal mass; Medical imaging; Pathology
Introduction
Digestive duplication is a rare congenital malformation that can affect any segment of the digestive tract [1] with a predominance of ileal involvement [2]. We report three cases of subdiaphragmatic digestive duplication, one gastric, the second duodenal, and the third jejunal; revealed by abdominal masses in boys aged 02 months, 20 months, and 5 years to describe the management of this pathology.
Case Series
Observation 1
A 2-month-old male infant entered the infant and neonatal visceral surgery department for abdominal mass progressing from birth, without associated transit disorder or vomiting. In his history, he had episodes of yellowish vomiting twice a day at the age of one month, which resolved spontaneously. The physical examination revealed an enlarged abdomen, a palpable renitent mass on the epigastric, right hypochondrium, and periumbilical regions. Laboratory tests were unremarkable apart from anemia at 9.7 g / 100 ml. The abdominal ultrasound revealed an intraperitoneal cystic mass without septa. The abdominal CT scan without and with contrast media confirmed the rather spherical cystic mass with purely liquid content, of a very regular outline, occupying the abdominopelvic cavity measuring 106 x 60 x 62 mm developed from the underside of the gastric antrum (Figure 1). Laparotomy was performed, showing the gastric origin of the cystic mass (Figure 2). Resection followed by gastro-duodenal anastomosis was performed. The postoperative follow-up was uneventful. The child was released from the hospital on the eighth day. The pathological examination of the excisional part confirmed a gastric duplication. The evolution was favorable for one year of follow-up.
Observation 2
A 5-year-old boy entered the emergency department for chronic epigastric pain. He had been having episodes of vomiting and constipation for two years. The clinical examination at the entrance revealed: a patient with no fever, an enlarged abdomen, with a renitent epigastric mass. Biology was unremarkable apart from anemia at 10.2 g / dl. Thoraco-abdominal CT scan revealed a large tubulo-spherical cystic mass at the expense of the duodenum, measuring 16 x 10 x 15 cm in favor of duodenal duplication (Figure 3). Laparotomy noted a cystic mass developing at the expense of the third portion of the duodenum. Resection of the cystic mass followed by a duodenojejunal anastomosis was performed. Postoperative course was unremarkable and no symptoms were detected during three months of follow-up.
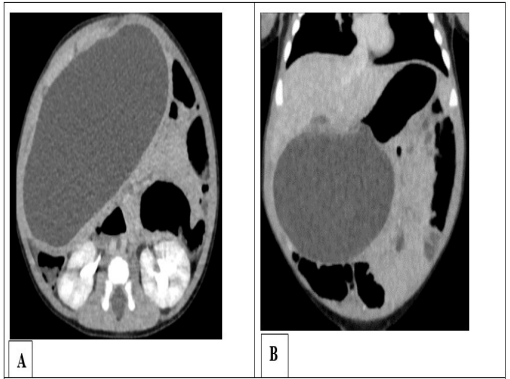
Figure 1: Abdominal CT scan with contrast media in axial sec- tion (A) and coronal reconstruction (B) showing an intraperi- toneal cystic mass developing from the underside of the gastric antrum, related to gastric duplication.

Figure 2: intraoperative photograph of the cystic mass at the expense of the stomach.

Figure 3: abdominal CT scan with contrast media in axial section (A) and sagittal reconstruction (B) showing a large tubulo-spherical cystic mass, developing at the expense of the duodenum.
Observation 3
A 20-month-old male infant was admitted with an abdominal mass evolving from the age of 12 months associated with episodes of constipation and yellowish vomiting. Upon entry, he was dyspneic with 90% SPO2 in ambient air. The lungs were free on auscultation. Abdomen was large with a renitent mass, occupying the right hypochondrium to the right flank on palpation. General condition was altered. He presented hydroelectrolyte disturbances with hyponatremia at 128 mmol / L and hypokalaemia at 3.2 mEq / L, a disturbance of renal function with serum creatinine at 110 µmol / L. The emergency abdominal ultrasound showed a large cystic spherical mass measuring 15 x 04 x 10 cm, containing hyperechoic images with reverberation artifacts compatible with gases, in favor of communicating digestive duplication. The abdominal CT scan confirmed the ultrasound data showing the rounded cystic mass with the presence of gas bubbles supernatant the liquid in the cyst (Figure 4). Surgical intervention was undertaken following an abdominal hyper pressure syndrome, revealing a large intraperitoneal cystic mass contiguous on the underside of the liver, in communication with the small bowel loop by a channel 30 cm downstream from the angle of Treitz (Figure 5). The cystic mass as well as the small segment in communication were resected, then an end-to-end jejuno-jejunal anastomosis was made. Anatomopathological examination of the excisional piece concluded in a jejunal duplication. The postoperative follow-up was uneventful and no complications were detected after two years.
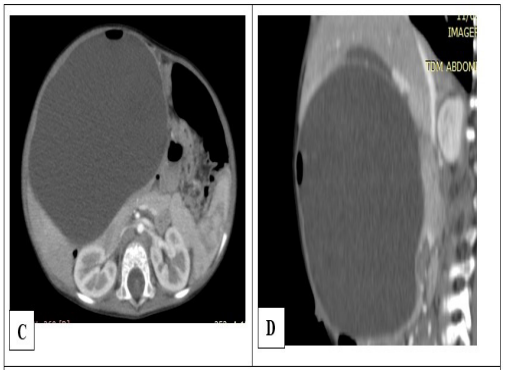
Figure 4: Abdominal CT scan with contrast media in axial sec- tion (A) and sagittal reconstruction (B) showing a voluminous, intraperitoneal cystic mass developing at the expense of the jejunum. Note the air bubble supernatant the fluid content wit- ness to the communication between the jejunal lumen and the lumen of the cyst.

Figure 5: Intraoperative photograph of the cystic mass attached to the underside of the liver (A) and attached to the jejunum (B).
Discussion
Digestive duplication, first described by Ladd around 1940 [3], is a rare congenital malformation that can affect all segments of the digestive tract from the oropharyngeal cavity to the anus [2]. While digestive duplications can be multifocal in 3% of cases, they are generally unifocal and predominate in the ileum followed by esophageal involvement [2,4] while our three cases are respectively gastric, duodenal, and jejunal topography. Digestive duplication occurs between the fourth and eighth weeks of embryonic life by the failure of the primitive intestine to channel and includes two types: communicating and non-communicating [5]. Even if male predominance, as in our series, is noted, there is no evidence for the existence of a relationship between gender, race, or genetic predisposition. Given the reported incidence of 1 in 4,500 births, the diagnosis and treatment of digestive duplication remain difficult [6]. Performing second-trimester obstetric ultrasounds can reveal cystic lesions if their size allows it. The clinical presentations of digestive duplication vary depending on its topography, size, and complications [6,7]. While most digestive duplications are symptomatic before the first two years of life, asymptomatic forms exist and will be discovered by chance or even very late [5-7]. The oropharyngeal or pharyngo-esophageal localizations are revealed early by severe respiratory disorders or dysphagia [7], while the gastric or intestinal topographies are manifested by digestive disorders such as nausea or vomiting or by distension or abdominal masses as evidenced by our three cases. Complications such as pain (recurrent) by the tension of the cyst, digestive hemorrhages, infection of the cyst, intussusception, extrinsic compression, or perforation can occur, motivating the surgical excision of these digestive duplications [7]. The disorders of the biological assessment which can present themselves are linked to these complications.
Radiologically, the antenatal ultrasound, of the second trimester, can show digestive duplication in the form of a cystic mass in contact with or even within a wall of the digestive tract. Postnatal, ultrasound should be the examination of choice in the diagnosis of digestive duplications except that it is limited for exploration of the thoracic esophagus and analysis of the organs of attachment of large lesions. In its uncomplicated form, digestive duplication is in the form of a fluid mass with anechoic content and whose wall reproduces the stratification of the layers of the carrier digestive tract with internal hyperechoic mucous layer and hypoechoic muscle external [2,7]. Two shapes can be distinguished, the tubular shape rarely and the spherical shape frequently. One of our patients presents a mixed form, tubular at its duodenal attachment then becoming spherical. As the ultrasound is a dynamic examination, it can identify peristaltic movements of the cyst wall if the probe remains fixed [7]. The hyperechoic image with reverberation artifact supernatant the fluid contents can be seen if the duplication communicates with the lumen of the carrier digestive tract. The contents of the cyst may be cloudy with debris and the lining altered in the complicated forms.
If digestive opacification is performed, it may show communication of duplication with the digestive lumen in communicating types and signs of compression in both types [2,8]. In large duplications such as our three cases, CT-scan can help in the diagnosis despite the limitations related to irradiation. Apart from the possibility of characterizing the wall of the lesion reproducing that of the carrier digestive tract, CT-scan makes it possible to assess the communicating type of the non-communicating by highlighting the hypodense air image supernatant the fluid content of the digestive duplication, as in one of our patients. This examination also makes it possible to specify the site of attachment of the lesion by the presence of an area with a thinner wall than the rest of the digestive tract at the place where the lesion appears but also the relationship of the lesion with the adjacent structures [2,7,8]. If MRI is non-irradiating, its limit lies in the invasive aspect with the need for sedation of children. However, it allows good lesion characterization like the scanner. Digestive duplication thus appears in the form of low signal intensity on T1-weighted images and very high intensity on T2-weighted images attached to the wall of a digestive tract [2,7].
The treatment is based on total surgical excision removing the adjacent portion of the carrier digestive tract depending on the topography of the lesion. The earlier the excision, the lower the morbidity and complications, hence the benefit of antenatal screening. Besides, even if the resection of asymptomatic forms remains controversial, the management of complicated forms is more difficult and requires more hospitalization time [7,9]. Also, there are forms of malignant transformation if the lesion is left in place or if the excision is not complete [2]. An exhaustive radiological assessment makes it possible, in addition to the differential diagnosis, to indicate a less invasive resection of unifocal digestive duplications, by thoracoscopic or laparoscopic route in uncomplicated forms [7].
Conclusion
Sub-diaphragmatic digestive duplications appear in the form of an abdominal mass and are revealed by digestive disorders or recurrent abdominal pain. Imaging can distinguish the spherical cystic type from the tubular type. CT-scan helps to characterize the voluminous forms, to differentiate the communicating and non-communicating type, and makes it possible to establish the relations of the lesion with the adjacent structures. Treatment consists of complete resection to prevent unforeseeable complications and reduce the risk of malignant transformation.
References
- Koo EJ, Jung HR, Jung E. Respiratory epithelium in an enteric duplication cyst at the ileocecal valve. J Pediatr Surg Case Rep, 2020; 59: 101521.
- Hur J, Yoon CS, Kim MJ, Kim OH. Imaging features of gastrointestinal tract duplications in infants and children: from esophagus to rectum. Pediatr Radiol, 2007; 37(7): 691–699. DOI: 10.1007 / s00247-007-0476-3.
- Ladd WE, Gross RE. Surgical treatment of duplications of the alimentary tract: enterogenous cysts, enteric cysts, or ileum duplex. Surg Gynecol Obstet, 1940; 70: 295–307.
- Peng HL, Su CT, Chang CY, Lau BH, Lee CC. Unusual imaging features of completely isolated enteric duplication in a child. Pediatr Radiol, 2012; 42 (9): 1142–1144. DOI: 10.1007 / s00247-012-2380-8.
- Tjendra Y, Lyapichev K, Henderson J, Rojas CP. Foregut Duplication Cyst of the Stomach: A Case Report and Review of the Literature. Case Rep Pathol, 2016; 1–4. DOI: 10.1155 / 2016/7318256.
- Jeziorczak P, Warner B. Enteric Duplication. Clin Colon Rect Surg 2018; 31 (02): 127–131. doi: 10.1055 / s-0037-1609028.
- Sangüesa Nebot C, Llorens Salvador R, Carazo Palacios E, Picó Aliaga S, Ibañez Pradas V. Enteric duplication cysts in children: varied presentations, varied imaging findings. Insights Imaging 2018. doi: 10.1007 / s13244-018-0660-z.
- Nour M, Zerhouni H, Oubejja H, Erraji M, Ettayebi F. Gastric digestive duplication in an infant in a case report. Arch Pediatr, 2014; 21 (5): 487. DOI: 10.1016 / s0929-693x (14) 71747-6.
- Patiño Mayer J, Bettolli M. Alimentary tract duplications in newborns and children: diagnostic aspects and the role of laparoscopic treatment. World J Gastroenterol 2014; 20: 14263–1427. DOI: 10.3748 / wjg.v20.i39.14263.

