New Onset Diabetic Ketoacidosis: An Adverse Effect of Nivolumab Therapy
Michelle Cancel, Kevin Schrader*, Mingchen Song
Department of Internal Medicine, SIU school of Medicine, USA
Received Date: 20/01/2022; Published Date: 04/02/2022
*Corresponding author: Kevin Schrader, MD, Department of Internal Medicine, SIU school of Medicine, 815 South English Avenue, Springfield, IL 62704, USA
Abstract
Immunotherapy is used more often than ever before to treat aggressive cancers such as small cell lung cancer. Although immunotherapy has fewer side effects than traditional chemotherapy, it is important to be aware of the rare life-threatening side effects. Our patient was started on immunotherapy with Nivolumab for extended stage Small Cell Lung Cancer (SCLC). After initiation she presented to the intensive care unit (ICU) with severe Diabetic Ketoacidosis (DKA), interestingly without any history of diabetes, hyperglycemia or pancreatic metastasis. She received protocol driven DKA therapy with improvement in her symptoms and resolution of DKA. Insulin-Dependent Diabetes Mellitus (IDDM) from Nivolumab is a side effect that is rarely reported in literature, hence making our patient’s case highly valuable. We describe her treatment course as well as review 50 cases of DKA after treatment with Nivolumab for a variety of malignancies. The primary objective of this review is to evaluate if there could be a way to screen potential Nivolumab recipients for potential reactions, such as DKA.
Introduction
Nivolumab is a monoclonal antibody against programmed cell death receptor-1 (PD-1) [1] that is being used increasingly in patients with high grade cancers due to its relatively lower side effect profile and better patient tolerance. Although Nivolumab is associated with improved cancer outcomes, it can be associated with detrimental immune related side effects that increase short term mortality. With the use of immune checkpoint inhibitors, there is an increasing incidence of immune related adverse events [2,3], however, new onset autoimmune diabetes mellitus is still uncommon and likely underreported. From our literature review, we found fifty cases of DKA associated with Nivolumab. We describe a patient with extended stage SCLC who was started on Nivolumab after failure of systemic chemotherapy with Carboplatin and Etoposide, and six months into her treatment she was admitted to the ICU with DKA. Prior to this episode of DKA, she had no history of hyperglycemia or insulin use. PD-1 receptor antagonists have been associated with autoimmune endocrinopathies, including IDDM. DKA can be associated with a high mortality if it is not detected early and treated appropriately. Therefore, it is important for clinicians to be aware of the association between IDDM and Nivolumab so they can better educate and treat their patients.
Case Report
An 82-year-old female with COPD, hypothyroidism and extended stage small cell lung cancer with metastasis to the brain, bone and liver presented to the emergency room with a 1week history of polyuria, polydipsia and generalized weakness. She reported no previous history of similar symptoms. She was diagnosed with metastatic small cell lung cancer one year ago from a liver biopsy. She failed systemic chemotherapy and was started on Nivolumab six months prior to this admission. Upon arrival to the emergency department, she was tachycardic and tachypneic. Labs were notable for: blood sugar of 998 mg/dL, anion gap of 24, bicarbonate 10 mmol/L, pH 7.15, pCO2 30 mmHg, beta hydroxybutyrate 8.5 mmol/L. Her urinalysis was positive for ketones, glucose and protein. She was diagnosed with DKA and was admitted to the ICU. Her 8 AM cortisol was 10 mcg/dL, going against the diagnosis of hyperglycemia from ectopic ACTH secretion. Her Islet cell antibodies were positive, which confirmed the diagnosis of autoimmune diabetes mellitus. She responded well to protocol driven DKA management and was discharged home safely on a basal-bolus insulin regimen.
Discussion
Nivolumab is an immunotherapy agent that is frequently used in patients with renal cancer, advanced melanoma and lung cancer [3]. It is a monoclonal antibody to PD-1, which results in activation of T-cells that act against cancer cells [1]. While this has been shown to be an effective therapy agent, new adverse effects have been uncovered. Common side effects of Nivolumab include diarrhea, fatigue, rash, itchiness, infusion site reactions and less commonly autoimmune side effects such as diabetes mellitus have been reported [3]. After reviewing the literature, we found 50 cases of new onset autoimmune diabetes mellitus and DKA with Nivolumab use [1-26]. We compiled a table to compare among the 50 cases (Table 1).
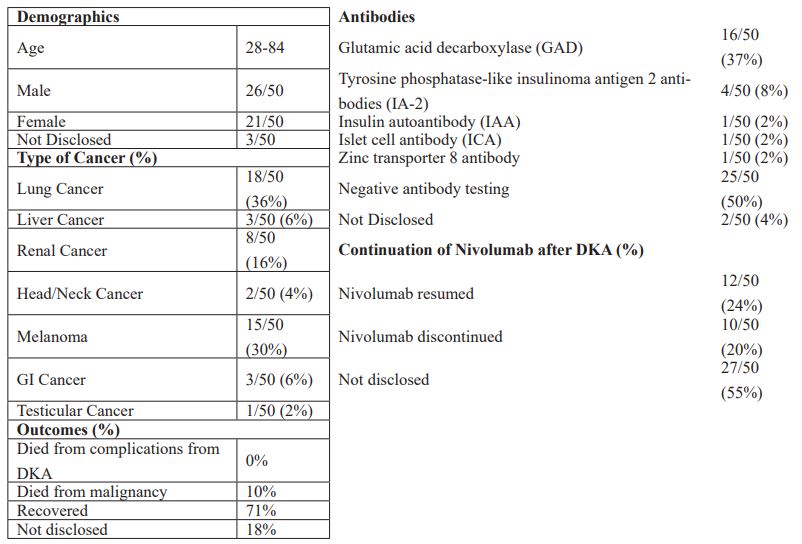
The patients were nearly evenly split, with 55% male and 44% female. They were predominantly lung cancer patients (36%), but also included melanoma (30%), renal cancer (16%), liver cancer (6%), and head/neck cancer (4%). Onset of symptoms from DKA varied from cycles 1 to 27 but most commonly presented after cycle 3 at 20%. The predominant autoimmune antibody in these cases was GAD (37%). Other antibodies present were IAA, IA-2, ICA, and ZnT8. However, 58% of the cases had negative antibody testing. Nivolumab was resumed in 24% of the cases and stopped in 20% (55% not disclosed). There were no reported cases of mortality from complications of DKA, 71% of cases recovered, but 10% did die from their primary malignancy. 18% did not disclose outcomes.
Our patient was a unique example of how Nivolumab can be associated with immune related adverse events, including endocrinopathies like IDDM. It is possible that our patient’s predisposition to autoimmune diseases such as hypothyroidism could have increased her likelihood of encountering another autoimmune condition. The PD-1 inhibition could have stimulated an autoimmune process that was in its dormant phase. Some of the cases reviewed did have other autoimmune endocrinopathies including hyperthyroidism and hypothyroidism. However, it does not appear to be a predisposing factor as the majority of patients with DKA after Nivolumab did not have any history of autoimmune endocrinopathies. It is unclear whether screening patients for predisposing antibodies prior to initiation of Nivolumab would reduce the risk of DKA in these patients. This review reveals some trends with regards to characteristics like type of malignancy treated with Nivolumab and antibodies present. However, there
is a wide variability of age range in patient cases as well as onset of symptoms? Also, the majority of patients had negative antibody testing. It would be difficult to pinpoint which patients as well as what time is best to test for hyperglycemia. What we take away from this review is that blood glucose monitoring should be explored in patients treated with Nivolumab therapy, but it is unclear if this would improve outcomes.
Conclusion
Nivolumab is a monoclonal antibody against PD-1 that is being used increasingly in patients with advanced malignancies. Although it is an effective drug, it can be associated with detrimental immune related adverse events. While new onset autoimmune diabetes is a rare side effect of Nivolumab, more cases are being reported every year. It is imperative to determine which patients are at greatest risk so that appropriate precautionary tools can be applied. Our hope is to explore strategies to better understand the association between Nivolumab and IDDM.
Ethics Statement
The authors of this manuscript attest that this clinical investigation was determined to not require
Institutional Review Board / Ethics Committee review. We did receive written consent by the
corresponding patient before pursuing publication.
References
- Tzoulis P, Corbett RW, Ponnampalam S, Baker E, Heaton D, Doulgeraki T, et al. “Nivolumab-induced fulminant diabetic ketoacidosis followed by thyroiditis.” Endocrinology, Diabetes & Metabolism Case Reports,
- Godwin J, Jaggi S, Sirisena I, Sharda P, Rao A, Mehra R, et al. “Nivolumab induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer.” Journal for Immunotherapy of Cancer, 2017; 5(40).
- Zaied A, Akturk H, Joseph R, Augustine S. “New onset insulin-dependent diabetes due to nivolumab.” Endocrinology, Diabetes & Metabolism Case Reports, ID: 2018; 17: 0174.
- Miyoshi Y, Ogawa O, Oyama Y. “Nivolumab, an Anti-Programmed Cell Death-1 Antibody, Induces Fulminant Type 1 Diabetes.” The Tohoku Journal of Experimental Medicine, 2016; 2(239): pp. 155-158.
- Harsch IA, Konturek PC. “Acute-onset diabetes mellitus with ketoacidosis in a nivolumab-treated patient with hepatocellular carcinoma.” Wiad Lek, 2018; 71(5): pp. 945-948.
- Marchand L, Paulus V, Fabien N, Perol M, Thivolet C, Saintigny P. “Nivolumab-Induced Acute Diabetes Mellitus and Hypophysitis in a Patient with Advanced Pulmonary Pleomorphic Carcinoma with Prolonged Tumor Response.” Journal of Thoracic Oncology, 2017; 12(11): pp. 182-184.
- Li L, Masood A, Bari S, Yavuz S, Grosbach A. “Autoimmune diabetes and thyroiditis complicating treatment with nivolumab,” Case Reports in Oncology, 2017; 10(1): pp. 230-234,
- Usui Y, et al. “Association of serum Anti-GAD antibody and HLA haplotypes with type 1 diabetesmellitus triggered by nivolumab in patients with non-small cell lung cancer,” Journal of Thoracic Oncology, 2017; 12(5): pp. 41-43.
- Teramoto Y, et al. “Case of type 1 diabetes associated with less-dose nivolumab therapy in a melanoma patient,” The Journal of Dermatology 2016 published online.
- Okamoto M, et al. “Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy,” Journal of Diabetes Investigation 2016; 7(6): pp, 915-918.
- Hughes J, Vudattu N, Sznol M, Gettinger S, Kluger H, Lupsa B. “Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy,” Diabetes Care, 2015; 38(4): pp. 55-57.
- Sakaguchi C, et al. “A case of nivolumab-induced acute-onset type 1 diabetes mellitus in melanoma,” Current Oncology vol, 2019; 26(1): pp. 115-118.
- Yilmaz M. “Nivolumab-induced type 1 diabetes mellitus as an immune-related adverse event,” Journal of Oncology Pharmacy Practice 2019 published online.
- Chokr N, Farooq H, Guadalupe E, “Fulminant diabetes in a patient with advanced melanoma on nivolumab,” Case Reports in Oncological Medicine 2018 published online.
- Lee S, Morgan A, Shah S, Ebeling PR. “Rapid-onset diabetic ketoacidosis secondary to nivolumab therapy,” Endocrinology Diabetes and Metabolism Case Reports 2018 published online.
- Venetsanaki V, Boutis A, Chrisoulidou A, Papakotoulas P. “Diabetes mellitus secondary to treatment with immune checkpoint inhibitors,” Current Oncology vol, 2019; 26(1): pp. 111-114.
- Zaied A, Lee A. “Nivolumab-induced autoimmune diabetic ketoacidosis,” CHEST, 2016; 150(4): pp. 255A.
- Tzoulis P, Stebbing J, Baker E, Heaton D, Corbett R. “Fulminant diabetic ketoacidosis complicating nivolumab immunotherapy,” Endocrine Abstracts, 2017; 49.
- Gauci M, et al. “Autoimmune diabetes induced by PD-1 inhibitor - retrospective analysis and pathogenesis: a case report and literature review,” Cancer Immunology Immunotherapy, 2017; 66(11): pp. 1399-1410.
- Capitao R, Bello C, Fonseca R, Saraiva C. “New onset diabetes after nivolumab treatment,” BMJ Case Reports
- Takahashi A, Tsutsumida A. “Fulminant type 1 diabetes associated with nivolumab in a patient with metastatic melanoma,” Melanoma Research, 2018; 28(2): pp. 159-160.
- Hao J, et al. “Development of type 1 diabetes after cancer immunotherapy,” AACE Clinical Case Reports, 2017; 3(3): pp. 242-245.
- Abid H, Watthanasuntorn K, Gnanajothy R. “Diabetic ketoacidosis: a rare side effect of Nivolumab induced insulin dependent diabetes mellitus,” Journal of the Endocrine Society, 2019; 3(1).
- Kapke J, Shaheen Z, Kilari D, Knudson P, Wong S. “Immune Checkpoint Inhibitor-Associated Type 1 Diabetes Mellitus: Case Series Review of the Literature and Optimal Management,” Case Rep Oncol, 2017; 10(3): pp. 897-909.
- Kumagai R, Muramatsu A, Nakajima R, Fujii M, Kaino K, Katakura Y, et al. “Acute-onset type 1 diabetes mellitus caused by nivolumab in a patient with advanced pulmonary adenocarcinoma,” J Diabetes Investig, 2017; 8(6): pp. 798-799.
- Sakurai K, Niitsuma S, Sato R, Takahashi K, Arihara Z. “Painless Thyroiditis and Fulminant Type 1 Diabetes Mellitus in a Patient Treated with an Immune Checkpoint Inhibitor Nivolumab,” Tohoku J Exp Med, 2018; 244(1): pp. 33-40.
- Olivares-Hernández A, Escala-Cornejo R, García-Domínguez A, Cruz-Hernández J. Severe Ketoacidosis as the First Clinical Manifestation of Type 1 Diabetes Mellitus Secondary to Immune Checkpoint Inhibitors, Asian Journal of Oncology, 2020; 6(02): 94-96. doi:10,1055/s-0040-1710147.
- Joseph M, Borikar M, Bursey D. Type 1 Diabetes Diagnosed in an 83-Year-Old Man After Nivolumab Therapy, J Endocr Soc, 2020; 4(Supplement_1). doi:10,1210/jendso/bvaa046,467.
- Marshall S, Kizuki A, Kitaoji T, et al. Type 1 Diabetes ACTH Deficiency and Hypothyroidism Simultaneously Induced by Nivolumab Therapy in a Patient with Gastric Cancer: A Case Report, Case Rep Oncol, 2020; 13(3): 1185-1190, doi:10,1159/000510044.
- Kyung K, Seok P, Hee C. Case of type 1 diabetes development after programmed cell death-1 inhibitor immunotherapy, Endocrine Abstracts, 2020. doi:10,1530/endoabs,70,aep359.
- Yaura K, Sakurai K, Niitsuma S, Sato R, Takahashi K, Arihara Z. Fulminant Type 1 Diabetes Mellitus Developed about Half a Year after Discontinuation of Immune Checkpoint Inhibitor Combination Therapy with Nivolumab and Ipilimumab: A Case Report, Tohoku J Exp Med, 2021; 254(4): 253-256. doi:10,1620/tjem,254,253.
- Baroud S, Mirza L. New-Onset Type 1 Diabetes Mellitus After Treatment with Nivolumab for Melanoma, Cureus, 2021. doi:10,7759/cureus,18679.
- Cristiano E, Grdinovac K, Bhattacharya R. Autoimmune Diabetes After Initiation of Nivolumab in a Patient with Hepatocellular Carcinoma, J Endocr Soc, 2021; 5(Supplement_1): A369-A370. doi:10,1210/jendso/bvab048,752.
- Yilmaz M. Nivolumab-induced type 1 diabetes mellitus as an immune-related adverse event, Journal of Oncology Pharmacy Practice, 2019; 26(1): 236-239. doi:10,1177/1078155219841116.
- Haque W, Ahmed S, Zilbermint M. Nivolumab-induced autoimmune diabetes mellitus and hypothyroidism in a patient with rectal neuroendocrine tumor, J Community Hosp Intern Med Perspect, 2020; 10(4): 338-339. doi:10,1080/20009666,2020,1771126.
- Feng C, Kibrik P, Castañeda C, Singh G. New-Onset Diabetic Ketoacidosis Secondary to Nivolumab Therapy in a Patient with Primary Central Nervous System Lymphoma, Osteopathic Family Physician, 2021: 40-43. doi:10,33181/13037.
- Mae S, Kuriyama A, Tachibana H. Diabetic Ketoacidosis as a Delayed Immune-Related Event after Discontinuation of Nivolumab, J Emerg Med, 2021; 60(3): 342-344. doi:10,1016/j, jemermed,2020,09,023.
- Keerty D, Das M, Hallanger-Johnson J, Haynes E. Diabetic Ketoacidosis: An Adverse Reaction to Immunotherapy, Cureus, 2020; 12(9): e10632, doi:10,7759/cureus,10632.
- Lo Preiato Valentina, Salvagni Stefania, Buganè Anna, et al, Nivolumab induced a rapid onset of diabetes mellitus in patients with cancer: three case reports, J Med Healthc Psychol, 2021; 1(1): 1–5.
- Delasos L, Bazewicz C, Sliwinska A, Lia NL, Vredenburgh J. New onset diabetes with ketoacidosis following nivolumab immunotherapy: A case report and review of literature, Journal of Oncology Pharmacy Practice, 2021; 27(3): 716-721, doi:10,1177/1078155220943949.

