The Effect of Short Term use of Lipiburn on Fat Loss in Obese Individuals: A Prospective Clinical Study
A. Bakr. M .Rabie*
Tyjito Biotechnology Ltd., 50 Wellington Street, 7th Floor, Central, Hong Kong, SAR, China
Received Date: 06/07/2020; Published Date: 27/07/2020
*Corresponding author:Bakr. M .Rabie, Tyjito Biotechnology Ltd., 50 Wellington Street, 7th Floor, Central, Hong Kong, SAR, China. E-mail: bkrabie@gmail.com
Introduction
The active ingredient in Lipiburn is Paeoniflorin (PF) which is one of the main effective components of the total glucosides of peony extracted from the root of Paeonia lactiflora [1]. The peony root extract has been used in traditional Chinese medicine for the treatment of a number diseases [2-4].
Rabie in 2019 reported the mechanism of action of Lipiburn on fat metabolism. Lipiburn’s mechanism of action was identified as over expression of Hormone Sensitive Lipase (HSL) through the activation of cAMP pathway [5]. HSL metabolises intracellular fat into the byproducts of fat, Fatty Acids and Glycerol. Micro array analysis demonstrated that, lipiburn enhanced the expression of Beta adrenergic receptors by 7.6 fold increase which in turn activated the cAMP to activate the Protein Kinase A (PKA). PKA added phosphate groups to the stored intracellular fat and that process activated the latent HSL [5].
Obesity is an epidemic in the United States of America as indicated in the 2007/8 National Health and Nutrition Examination (NHANES) Survey [6]. The prevalence of obesity amongst adults is over 70%. The impact of obesity on health care cost is approximately 42% higher for obese people as compared to non-obese individuals. With an increase of 6% to 7% every 10 years it presents a serious problem to health care [6-9].
The objectives of this prospective clinical study is
- To examine the effect of lipiburn on fat loss in obese
- To examine the impact of lipiburn on quality of life. This includes a patient's general well- being, restful sleep status, and self-perceived rate of
Study design, inclusion, exclusion criteria and results section are presented here as was presented to us by Dr. Ghaly with minor amendments as per my understanding of how the study was conducted on my companies behalf.
Clinical Study Design
“A prospective cohort study is a longitudinal cohort study that follows over time a group of similar individuals (cohorts) who differ with respect to certain factors under study, to determine how these factors affect rates of a certain outcome “[10]. The current clinical study fits the definition of prospective clinical study. The group of similar individuals in this study is the obese patients with 35-40% BMI who were followed for 8 consecutive weeks making this study a longitudinal cohort study.
Dr. Fouad I. Ghaly, MD, FCCP, ABAAM, AABRM conducted the clinical study at the Ghaly Centre for Regenerative Medicine, a regenerative medicine clinic located at 20911 Earl Street, Suite 260, Torrance, CA 90503. Obese individuals were recruited to participate in this prospective clinical study. Participants were asked to use Lipiburn supplement for 8 consecutive weeks. 10 participants were randomly selected from the larger recruited group. Those 10 patients must have recently completed the 8-week course of LipiBurn supplementation, and have completed a Healthy Days Core Module Questionnaire (CDC HRQOL– 4). Dr. Ghaly was totally independent with no input from us or any other funding source in the design, conduct, and presenting the data of the current clinical study.
Inclusion Criteria
Each participant signed a written informed consent. Participants had to be 35 years of age or older, willing to complete post-treatment questionnaires, and a BMI of 35-40 (minimal standard deviation).
Exclusion Criteria
Persons were excluded if they had a history of serious disease or illness, including cancer. In addition, persons were excluded if they had not used LipiBurn for 45 consecutive days prior to review participation; abused alcohol or drugs; or were pregnant or lactating.
Intervention
Participants of this study were asked to take 6 capsules of Lipiburn per day, 2 capsules after each meal. Prospectively, patients were asked to go for eight consecutive weeks of weigh-ins and data collection. Lifestyle interventions, such as diet and exercise, were not specifically altered during the study period [8]. Additionally, participants were asked to complete a Healthy Days Core Module Questionnaire (CDC HRQOL– 4) upon commencement and at the end of supplementation. Participants were then randomly selected on or about the fifty-fifth (55th) day after beginning a course of LipiBurn supplementation at their own discretion, and completing a Healthy Days Core Module Questionnaire (CDC HRQOL– 4) upon commencement.
Sample Demographics
The 10 randomly selected patients were comprised of 7 women and 3 men between the ages of 35-65 years old.
Results
Table 1: Weight Outcomes and Results of Review Group.
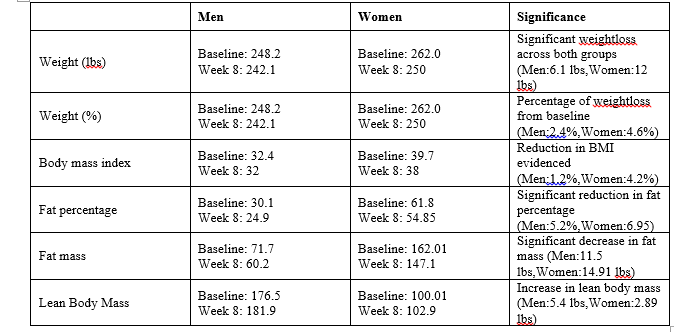
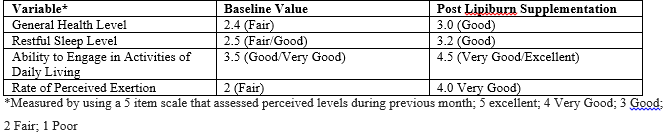
Table 2: LipiBurn Supplementation Effect on Health Level, Sleep, Quality-of-Life, and Rate of Perceived Exertion Data According to Review Group.
Discussion
Results of this prospective clinical trial indicated that the use of lipiburn resulted in significant weight loss in both men and women when taken orally for 8 weeks period. Our earlier research indicated that Lipiburn increases the ability of human adipocytes to break down triglycerides into Glycerol and fatty acids through over expression of HSL [5]. HSL is under tight hormonal and neuronal control and was first described by Hahn et al [11]. Variations in HSL expression probably modulate the extent of adipose tissue lipolysis. HSL- stimulating hormones, like catecholamines, activate this enzyme through cAMP-dependent phosphorylation [4]. We demonstrated that Lipiburn activated the HSL through activation of the cAMP pathway [5]. Furthermore, our microarray analysis demonstrated a 7.6 fold increase in the Beta adrenergic receptors responsible for fat metabolism [5]. In the current study, participants continued to eat their normal diet and resumed their normal daily activities. Therefore, it is safe to say that the fat loss was due to over expression of the beta adrenergic receptors leading to significant increase in HSL levels expressed as a result of lipiburn intake.
Lipolysis leads to the release of Fatty acids which could provide an excellent source of energy. Such extra energy positively impacted quality of life measures in the sample (Table 2). The released Glycerol as a result of taking lipiburn is water soluble, hence, it was recommended in the current trial that volunteers drink two litres of water per day to flush out the released Glycerol. Clinically, men lost over 6 pounds and women lost 12 pounds in 8 weeks after taking 6 capsules/ day after meals (table 2). It is important to point out that none of the participants were instructed to go on a special diet or alter their existing daily exercise. Exercise increases the amount of HSL [12]. Intermittent fasting lowers glucose levels in the blood which reduces the released insulin levels. Insulin inhibits phosphorylation by hydrolysis of cAMP [13] pointing to a converse relationship between insulin and levels of HSL. Since participants of the current study were not instructed to adopt a special diet or a specific exercise protocol, then the fat loss experienced must be due to the Lipiburn intake.
Measurement of fat loss indicated 5.2% reduction and 6.95% reduction in body fat in men and women respectively (table 1). Confirming the importance of lipiburn in ultimately over expressing HSL. It is very promising and certainly warrants much larger clinical trial to note the significant reduction in fat mass being 11.5 pounds reduction in men and 14.91 pounds reduction in women ( table 1). In a clinical study on 10 healthy Korean volunteers the pharmacokinetics of Peony root extract were identified after oral intake of 3.4 grams daily. Only 0.2 nanogram was identified after 12 hours indicating total absorption and clearance [14]. Li et al., 2016 carried out a clinical trial to identify the safety and tolerability of PF after single and multiple intravenous injections [15]. The group reported that even at 9 grams concentration, PF was safe and no adverse effects were reported. Our data in conjunction with the above report point out to the fact that lipiburn is safe and effective supplement that leads to significant reduction in fat mass in both men and women (table 1). An additional value of Lipiburn is that it helps with a life style change. From my personal experience using Lipiburn, The steady reduction in fat mass motivated me to eat less, skip a second serving of desert and motivated me to start exercise and lead a much healthier life style.
This brings us to the effects of the released byproducts of all this broken down fat on quality of life. This study aimed to investigate if short term provision of LipiBurn improves functional status parameters. The impact of this nutritional supplement on quality of life is also examined. This includes patient's general well-being, restful sleep status, and self-perceived rate of exertion. Analysis of data from the questionnaire showed that the ability to Engage in Activities of Daily Living went from a base line of 3.5 (good) to 4.5 (excellent). Similarly rate of perceived exertion to meet daily activities went from 2 being poor to 4.5 indicating excellent performance. This apparent improvement of general performance could be in part due to the release of fatty acids as a result of breaking down triglycerides [5]. Furthermore, Ghayur et al, at McMaster University studied the cardiovascular and airway relaxant activities of peony root extract and demonstrated that PF extract possess cardio suppressant, vasodilatory, anti-platelet aggregation, and tracheal and airway relaxant activities [16]. Vasodilatation means more blood being delivered throughout the body which also means more oxygen being delivered. This could explain the perceived significant improvement in the ability to engage in daily activity ( table ). In 2008 my team reported a close association between Obesity and severity of Obstructive Sleep Apnea (17). It is possible that higher oxygen levels reported by Ghayer could be a contributing factor to the improvement in the restful sleep levels seen in the current clinical study [16]. In conclusion, Lipiburn supplement is safe and leads to significant fat reduction and weight loss in both men and women. It also provides extra energy through breaking down fat into the byproducts of fat being Glycerol and fatty acids. Lipiburn leads to a significant improvement in quality of life through improving several functional status parameters.
Acknowledgement
We thank Dr. Fouad Ghaly, At the Ghaly Centre of Regenerative Medicine in LA, California for independently carrying out all aspects of this clinical trial on behalf of Tyjito Biotech ltd.
References
- Foster S, Yue CX. Herbal emissaries: bringing Chinese herbs to the west. Healing Arts Press, Rochester, Vermont. 1992.
- He DY, Dai SM. Anti-inflammatory and immunomodulatory effects of Paeonia Lactiflora Pall., a traditional Chinese herbal medicine. Front Pharmacol. 2011;2:1-5.
- Tan J, Zhao Q, Yang L, Shang Z, Du Z, Yan M, et al. Chemical constituents in roots of Paeonia lactiflora. 2010;41:1245-1248.
- Zhang X, Wang J, Li X, et al. A study on the chemical constituents of Paeonia lactiflora Pall. Shengyang. 2001;18:30-32.
- A. Bakr M. Rabie, The mechanism of action of Lipiburn on fat metabolism. Frontiers in Bioscience. 2019;24(3):427-434.
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999– 2008. Journal of the American Medical Association. 2010;303(3):235-241.
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer- and service- specific estimates. Health Affairs. 2009;28(5):w822-w831.
- World Health Organization. Obesity and Overweight. Geneva: WHO; 2013. URL: http://www.who.int/ mediacentre/factsheets/fs311/en/ (accessed August 2014).
- Centers for Disease Control and Prevention, Behavioral Risk Factor Surveillance System, http:// www.cdc.gov/brfss/.
- Definition of prospective cohort study-NCI Dictionary of Cancer Terms.
- Hahn P. The postnatal development of hormone sensitive lipase in brown and white adipose tissue of the rat. Experientia. 1965;21:634-635.
- Enevoldsen LH, et.al. The effect of exercise training on hormone-sensitive lipase in rat intra-abdominal adipose tissue and muscle. 2001;3:871-877.
- S. Meijssen M. Castro Cabezas C. G. M. Ballieux R. J. Derksen S. BilecenD. W. Erkelens. Insulin Mediated Inhibition of Hormone Sensitive Lipase Activity in Vivo in Relation to Endogenous Catecholamines in Healthy Subjects.. The Journal of Clinical Endocrinology & Metabolism, 2001;86(9):4193-4197. https://doi.org/10.1210/jcem.86.9.7794.
- Seo-Hee, Heo Dong-Seok, Lee Seong-Ho, Ham Jung-Hee, Cho Young-Dal, Kwon Yong-Bok, et al. Pharmacokinetic evaluation of paeoniflorin after oral administration of Paeoniae Radix extract powder to healthy Korean subjects. Journal of Pharmaceutical Investigation. 2016;46(3):pp 273-282.
- Li X, et al. Pharmacokinetics, Safety, and Tolerability of Amygdalin and Paeoniflorin After Single and Multiple Intravenous Infusions of Huoxue-Tongluo Lyophilized Powder for Injection in Healthy Chinese Volunteers. 2016.
- Ghayuer et. al. Cardiovascular and airway relaxant activities of peony root extract. Canadian Journal of Physiology and Pharmacology. 2008;86(11):793-803
- Huie Ming Hou, et al. Dentofacial Characteristics Of Chinese OSA in Relation to Obesity and Severity. Angle Orthodontics. 2006;76(6):962-9.

