Correlation of BMI, Age, gender to NGT, IFG, and IGT during Oral Glucose Tolerance Test (OGTT) conducted at The University of Chenab
Aqna Malik1,2,*, Atifa shafqat2, Tehreem Sajjad2, Wajiha Noor2 and Ayesha Tariq2
1Department of Pharmacy, University of Chenab, Gujrat
2Department of Pharmacy, University of Lahore, Lahore
Received Date: 12/05/2025; Published Date: 19/06/2025
*Corresponding author: Dr. Aqna Malik, Department of Pharmacy, University of Chenab, Gujrat; Department of Pharmacy, University of Lahore, Lahore.
Web of Science Researcher : IDKIE-3543-2024
ORCID: https://orcid.org/0000-0003-3875-9621
Abstract
Background: The fasting blood glucose (FBG) level (collected after an 8 to 10 hr fast) is used to screen for and diagnose diabetes. An oral glucose tolerance test (OGTT / GTT) may also be used to diagnose diabetes. To be certain of a diagnosis of diabetes, two tests (fasting glucose or the OGTT) should be done at different times. The OGTT involves a fasting glucose measurement, followed by the patient drinking a glucose drink to 'challenge' their system and another glucose test 2 hours later. It is used to diagnose diabetes mellitus when previous investigations have indicated a fasting glucose of 6.1-6.9 mmol/L.
Aim: Screening for type 2 DM or prediabetes among non diabetic persons before any other symptoms
Objective: To find the Correlation of BMI with age, FBG baseline, and FBG 2hr regarding impaired glucose tolerance.
Methodology:
Study Design: An undergraduate lab-based test was performed using a blood glucometer.
Study Settings: The study was designed to conduct the OGTT test in the Pharmacology LAB. Students of the 4th semester
Verbal consent was obtained from students one day before the test. Previous medical history was recorded, and students were asked if using any medication or not. Students were asked to fast overnight of 8-14 hr (water is allowed) before the morning test. List of students who have participated in OGTT. The OGTT test was conducted as follows.
Results: Among 35 students, the mean age (20.7±0.79) of the Pharmacology Lab who took part in the OGTT experiment. 3(8.57%) were males and 32(91.42%) were females. The BMI (22.3±4.21) of all 35 students was calculated from the weight and height data of the class.7 (20%) participants have Type 2 DM in their families, either from their mother or father. Normal glucose tolerance (NGT) was observed in 23 (65.71%) of 35 students.3 (8.57%) students, depicted normal but high response. 1 (2.857%) Student showed Sustained, high FBG levels which may be possibly related to impaired glucose tolerance (IGT). While 6(17.14%) females exhibited impaired fasting glucose (IFG) with different indices. Except for one student, all students have BMI less than 25 kg/m2. Among other risk factors, 7 students have Type 2 DM in their families.
Conclusion: The present study underlines a significant positive link between BMI and 2-hour post-load FBG, hence underlining the relationship between more adiposity and worse glucose tolerance. Increased BMI may be linked with postprandial glucose responses that appear stronger. suggesting that postprandial assessments could be more sensitive markers of metabolic risk in overweight individuals. The function of BMI in reactive hypoglycemia is yet unknown and has to be further researched. The findings imply that 2-hr FBG values should be included in risk classification for glucose metabolism disorders, particularly in people with high BMI Comparing between genders, it was observed that Females might show more insulin sensitivity than males, as seen in the above study, in early adulthood, hence influencing different RH or IGT prospective
Keywords: OGTT; Impaired Glucose Tolerance; Reactive Hypoglycemia; Impaired Fasting Glucose
Background
The accurate quantification of glycemia through reliable and feasible tests for screening and early detection has historically posed significant challenges. The indications for conducting the OGTT are varied [1]. The Oral Glucose Tolerance Test (OGTT) has undergone significant evolution over the past century. Changes include variations in glucose solution concentration for testing (50, 75, or 100 g), the use of plasma glucose rather than whole blood, adjustments in sample collection timing (0, 30, 60, 90, 120 minutes), the number of samples needed for diagnosis, and the criteria and terminology used for diagnosing dysglycaemia, such as prediabetes, chemical, borderline, subclinical, latent, or overt diabetes [2]. Plasma glucose concentrations, assessed following an overnight fast or glucose loading, have served as the primary method for diagnosing type 2 diabetes mellitus for over a century. There was insufficient agreement on the appropriate cut-points for glucose loading to diagnose prediabetes and type 2 diabetes mellitus, necessitating further re-evaluation.
There is ongoing debate regarding the diagnostic criteria for Type2DM) and the role of OGTT in clinical practice. The FPG was not consistently employed for the diagnosis of T2DM. The index of glucose excursions, including the glucose curve shape and area under the curve (AUC), was proposed early in the development of the OGTT, with recent adaptations. The threshold levels of these intermediate values were comparatively lower than those currently endorsed, resulting in a higher prevalence of detected diabetes. In the earlier era of the OGTT, prediabetes was not defined.
By the end of the 1960s, it was generally recognized that reliance solely on the FPG for diagnosis resulted in identifying individuals at a late stage in the natural progression of diabetes. This led to the formulation of at least 6 distinct recommendations for oral glucose loads, ranging from 50 to 100 g. Subsequently, the American Diabetes Association (ADA) proposed the estimation of body surface area to determine the appropriate glucose load for the (OGTT), with dosage calculations not grounded in ideal body weight or body surface area [3,4].
In 1980, the WHO recommended the global standardization of the (OGTT) using a 75- glucose load, which remains in use today. The US National Diabetes Data Group (NDDG) and the World Health Organization (WHO), set (FPG ≥ 7.8 mmol/L and 2h PG ≥ 11.1 mmol/L) for the diagnosis of diabetes standard value for 2h PG is supported by studies on diabetic retinopathy; however, the optimal value for diagnosing FPG lacks adequate standardization.
In 1997, an ADA Expert Committee recommended lowering the cut-off for the FPG from ≥ 7.8 mmol/L to ≥ 7.0 mmol/L for the diagnosis of diabetes [4]. The thresholds were established to address the discrepancy between the 2-hour plasma glucose (PG) and fasting plasma glucose (FPG), acknowledging that numerous individuals may present with a 2-hour PG ≥ 11.1 mmol/L and/or an FPG < 7.8 mmol/L. This approach also aims to streamline the diagnostic process between the FPG and the oral glucose tolerance test (OGTT) [5].
In 1999, the WHO revised the cut-off point for fasting plasma glucose (FPG) to ≥ 7.0 mmol/L while maintaining the 2-hour plasma glucose (PG) threshold for the diagnosis of type 2 diabetes mellitus (T2DM) [6].
Carbohydrate metabolism deficiencies, including insulin resistance (IR), hyperinsulinemia, prediabetes, and type 2 diabetes, are quickly emerging as a significant and pervasive health issue affecting all social strata [7]. Furthermore, reactive hypoglycemia denotes hypoglycemic symptoms and diminished blood glucose levels occurring in non-diabetic individuals. Patients within 2–5 hours postprandially may be associated with the aforementioned issues. In recent years, there has been a notable rise in the number of persons exhibiting symptoms indicative of hypoglycemia being referred to medical practitioners and/or nutritionists
Impaired fasting glucose (IFG) [fasting plasma glucose (FPG), 100 to 125 mg/dL] is a prediabetic condition. Individuals with impaired fasting glucose (IFG) exhibit a fivefold increased risk of advancing to type 2 DM compared to those with normal glucose tolerance (NGT). Prior clinical investigations (2–8) have recorded various metabolic irregularities in individuals with impaired fasting glucose (IFG), including hepatic insulin resistance and compromised first-phase insulin production. Conflicting findings have been published about insulin sensitivity in the skeletal muscle of individuals with impaired fasting glucose (IFG). Certain investigations have indicated normal or nearly normal insulin sensitivity in skeletal muscle [8].
During the course of type 2 DM, poor glucose tolerance and impaired fasting glucose constitute an intermediate stage. It occurs when patients' glucose levels range from 100 to 125 mg/dL (5.6 to 6.9 mmol/L) while fasting, or when their 2-hr glucose levels range from 140 to 199 mg/dL (7.8 to 11.0 mmol), it affects about 10% to 15% of American adults. Although they are higher than normal, these glucose levels do not yet meet the criteria for diabetes. Primary prevention efforts should focus on patients with impaired glucose tolerance or impaired fasting glucose because of the high risk of diabetes that they pose. Polycystic ovarian syndrome PCOS , a history of gestational diabetes or a baby with greater size than his gestational age, a sedentary lifestyle, hypertension, dyslipidaemia, and a family history of diabetes are all risk factors for developing diabetes [8].
Pathological development from normal glucose tolerance to type 2 diabetes include dual deficiencies, like insulin resistance and an insulin secretory defect due to β-cell malfunction [9]. A significant compensatory hyperinsulinemia and reduced insulin sensitivity are hallmarks of insulin resistance. Plasma glucose levels are initially kept within the normal range. A decrease in β-cell secretory capability is observed in those who would subsequently acquire diabetes. When insulin secretion drops during the initial phase of the glucose tolerance test, the result is an increase in postprandial glucose levels. Fasting glucose levels rise as β-cell function continues to deteriorate with age. Increased secretion of insulin leads to the development of diabetes [10]. Being overweight or obese based on body mass index (BMI) is linked with high FBG across different regions [11,12].
AIM: Screening for type 2 DM or prediabetes among non diabetic persons before any other symptoms
Objective: To find the correlation of BMI with age, FBG baseline, and FBG 2hr regarding impaired glucose tolerance
Methodology
Study Design: An undergraduate lab-based OGTT was performed using a blood glucometer.
Study Settings: The study was designed to conduct the OGTT in the Pharmacology LAB.
Sample size: 35 Participants were students of the 4th semester who took part voluntarily
Verbal consent was obtained from students one day before the test. Previous medical history was recorded, and students were asked if using any medication or not. Students were asked to fast overnight for 8-14 hr (water is allowed) before the morning test. List of students who have participated in OGTT. The OGTT test was conducted as follows.
Data Collection Procedure:
- The OGTT test was performed using a glucometer with glucometer strips.
- An ACCU-CHECK Glucometer with ACCU-CHECK strips was used to take baseline values from all students.
- Each time Blood was drawn, students were asked to wipe their finger using an alcoholic cotton swab.
- The ACCU-CHECK Glucometer was used to prick the finger of the subject with a small device called a lancet.
- A drop of blood was applied to a test strip, and it was inserted into an ACCU-CHECK Glucometer, which measures the glucose in your blood was performed at 30,60,90, and 120 minutes later.
- Time at which baseline fasting blood glucose levels were obtained, termed as “0” time.
- Students were asked to rest during the test.
- Each student was given 75g of glucose dissolved in 250 to 300 mL of water and ingested over 5 minutes [13].
- (A commercial, more palatable form of glucose can be ingested, but the use of anhydrous or monohydrate form of glucose is still in question)
Data Analysis:
Inclusion&Exclusion Criteria:
Inclusion Criteria: Both male & female students of the 4th semester who took part voluntarily
Exclusion Criteria: Any person outside this class
Ethical Consideration: The study was approved by the ethical committee of The University of Chenab.
Statistical Analysis: GraphPad version 10.4.2. was applied to find correalation between BMI,Age and FBG levels among Partcipants.
Results
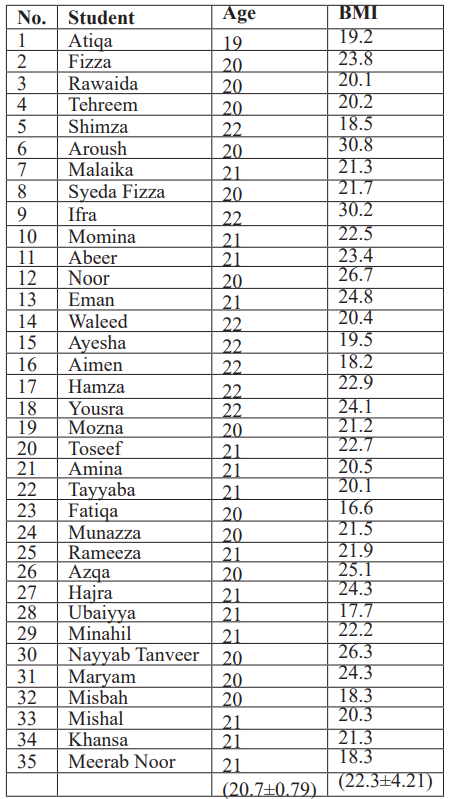
Demographics of Participant students: 35 of mean age (20.7±0.79) students of Pharmacology Lab took part in OGTT experiment. 3(8.57%) were males and 32(91.42%) were females
Variation of BMI among Participants: The BMI (22.3±4.21) of all 35 students was calculated from the weight and height data of class.
Table 1: Age and BMI among Participants.
Family History of Type DM among Participant Students
Among 35 student participants, 7 (20%) participants have Type 2 DM in their families, either from their mother or father. 6(85.7%) out of 7 were females and 1(15%) male participant. Among 35, one student, Munazza, who has diabetes in her family, 101→ 91→ 89→ 89→ 89 depicted IFG with odd decline with possibly reactive Hypoglycemia.
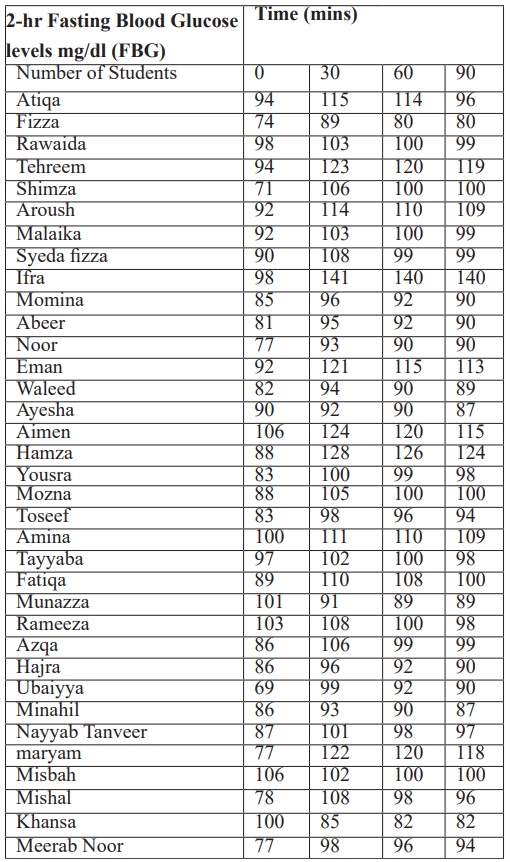
Post-OGTT FBG levels obtained: Post-OGTT FBG levels obtained at time 0,30,60. 90,120 mins are tabulated in Table
Table 2: Post-OGTT FBG levels OGTT.
Guidelines adopted for Interpretation of Blood Glucose Levels [14]:
OGTT helps to detect diabetes and IGT [15].IGT is more often linked to risk factors and events for cardiovascular disease than IFG, and it also predicts diabetes [16]. Results are categorized based on following classification of FBG levels
- Fasting:70 to 99 mg/dL (3.9 to 5.5 mmol/L)
- Random (any time of day):≤125 mg/dL (6.9 mmol/L)
- Before meals (for people with diabetes):80 to 130 mg/dL
- 1-2 hours Postprandial (for people with diabetes):≤180 mg/dL
Prediabetes: FBG levels between 100 and 125 mg/dL (5.6 to 6.9 mmol/L) [17].
Diabetes: FBG levels of 126 mg/dL (7.0 mmol/L) or higher on two separate tests [18].
- FBG levels between 100 and 125 mg/dL (5.6 to 6.9 mmol/L).
- FBG levels 126 mg/dL (7 mmol/L) or higher on two separate tests, confirm diabetes.
Impaired glucose Levels during OGTT
Patients with impaired glucose tolerance (IGT) or impaired fasting glucose (IFG) are at significant risk for diabetes
Elevated fasting glucose Levels (100-125 mg/dL) → possible IFG
Abnormally high peak or sustained glucose (140-199 mg/dL) → possible IGT
General OGTT Interpretation of Blood glucose levels Post OGTT
All the Observations regarding the OGTT Interpretation of Blood glucose levels post-OGTT were recorded in Tabular and Graphical form and interpreted in the Table:
Table 3: OGTT Interpretation of Blood glucose levels Post OGTT.
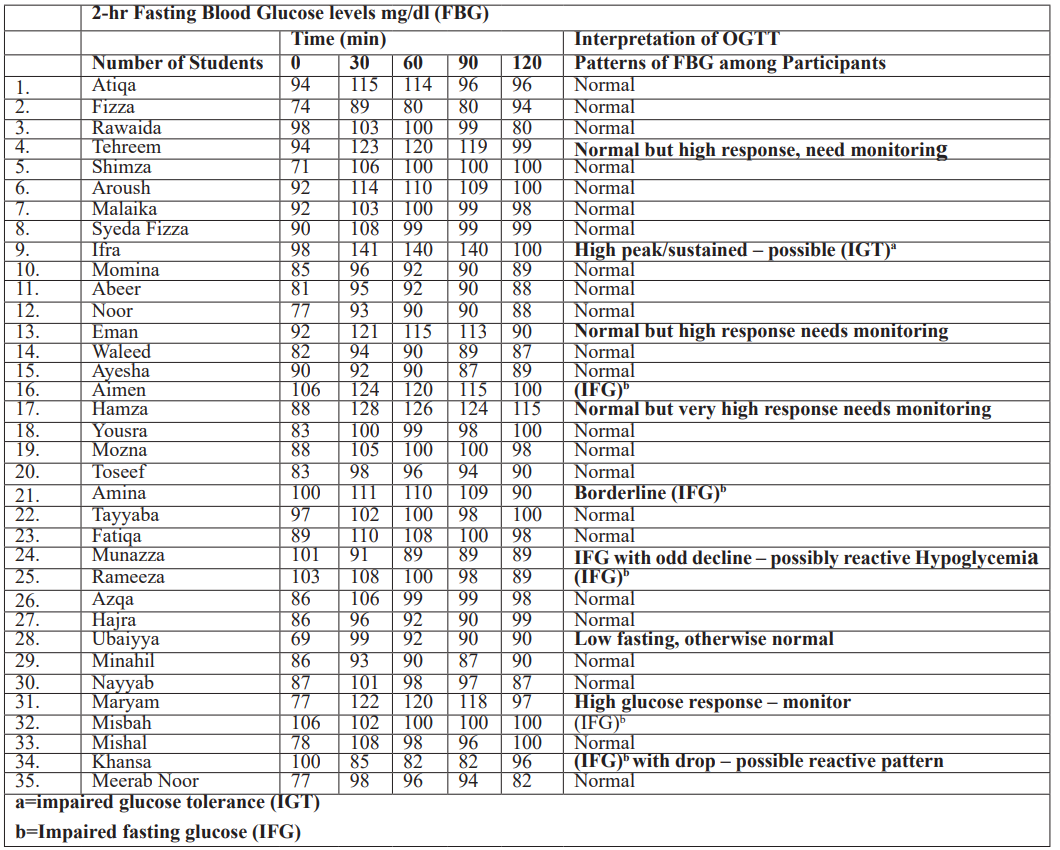
Normal Glucose Tolerance (NGT)
Normal rise and gradual return towards baseline were observed in 23 (65.71%) among 35 students. They exhibited physiological curves with normal rise and gradual return toward baseline with moderate peaks and drops by 120 min.
Atiqa, Fizza, Rawaida, Shimza, Malaika, Syeda Fizza, Momina, Abeer, Noor, Waleed, Ayesha, Yousra, Mozna, Toseef, Tayyaba, Fatiqa, Azqa, Hajra, Minahil, Nayyab, Mishal. Meerab Noor
Exception: Aroush: (92 → 114 → 110 → 109) – Mildly elevated but steady return.
Gender wise Variation regarding Normal Glucose Tolerance (NGT)
Normal rise and gradual return towards baseline were observed in 2 (66.66%) males and 21(91%) females.
Consistently low values with modest rises
However, among those 23 participants, 8 females (34.78%) students Abeer, Noor, Waleed, Ayisha, Toseef, Mouzna, Ubaiyya, Meerab Noor depicted consistently low values with modest rises. No male showed above pattern.
Normal but high response, need monitoring
Among 35, 3 (8.57%) students, depicted normal but high response, including 2(66.6%) females and 1 male However
1(11.66%) student among 3 showed slightly high but within the tolerance zone. EMAN (92 → 121 → 115 → 113→90)
High values sustained throughout may suggest delayed glucose clearance [19]
1 (2.857%) Student IFRA (98 → 141 → 140 → 140) among 35 has shown a high peak/sustained, which may be possibly related to impaired glucose tolerance (IGT), which was introduced in 1979, is an intermediate category spanning the grey area between clear diabetes mellitus and risk-free, more normal glucose tolerance (Rynders et al., 2014). Those at high risk of later development of non-insulin-dependent diabetes mellitus (NIDDM) but low risk of particular diabetic problems made up the IGT group. Though even those shown to be IGT only once are at higher risk of developing NIDDM, the unpredictability of the oral glucose tolerance test makes categorizing people as IGT difficult [20].
Impaired glucose Levels during OGTT
Among 35 participant students, 6(17.14%) females exhibited impaired fasting glucose (IFG) categorized into following depending on FNG level
Borderline IFG [21]
Among 6 participant students exhibiting impaired fasting glucose (IFG) 1 female student
Amina (100→ 111→ 110→ 109→90) need extra care as her FBG levels were borderline.
IFG with odd decline – possibly reactive Hypoglycemia
Among 6 participant students exhibiting impaired fasting glucose (IFG) 1 student showed odd decline depicting possibility of reactive hypoglycemia Munazza (101→91→89→89→89)
IFG with drop – possible reactive pattern
Among 6 participants 1(16.66%) female student depicted IFG with dropped pattern which possibly can be a reactive pattern
Khansa (100→85→82→82→96)
Diabetic Range: No student had diabetic range FBG ≥200mg/dl during 2-hour post OGTT.
Statistical Analysis
Correlation of BMI with Age, FBG baseline, and FBG 2hr: Based on FBG levels, the Correlation of BMI with Age, FBG baseline, and FBG 2-hr was found via Pearson’s correlation using GraphPad 10.4.2. Pearson correlation was found to have High degree values ranging from ±0.50 to ±1, suggesting a strong correlation between NGT, NGT with sustained High FBG levels, IFG &IGT. BMI above 25 kg/m2 were at increased risk of high FBG. Participant IFRA with BMI 30.2 grouped in Obese (Class I), depicted IFG. While participating in other groups depicted a normal range BMI like NGT (21.95±3.19), NGT with sustained High FBG levels (23.1±2.06), and IFG (20.7±1.12). Possible reasons for impaired glucose tolerance in NGT with sustained high FBG levels and IFG groups may be family history.
The RM one-way ANOVA test also proved significant p<0.0001.
Table 4: Correlation of BMI with Age, FBG baseline, and FBG 2hr.

Discussion
The current study sought to investigate the relationship between body mass index (BMI), age, fasting blood glucose (FBG) baseline, and (FBG 2hr) regarding reactive hypoglycemia (RH) and impaired glucose tolerance in Post OGTT performed in the Pharmacology LAB, (IGT). Results showed clear trends that highlight the dynamic interaction between anthro1spometric indices and metabolic factors.
BMI and FBG 2hr showed a positive link, implying that those with BMI above 25 kg/m2 would be more prone to have increased postprandial FBG levels. As in above study one student was found to be highly prone to IGT. Many researchers found that insulin resistance, a characteristic of IGT, is linked with obesity, especially central adiposity. Delayed glucose clearance in people with more body fat could be explained by insulin's reduced capacity to work effectively, which would then lead to increased 2-hr FBG levels post-OGTT. This can be clear proof of prediabetes as screening for Type 2 DM [23].
On the other hand, the relationship between BMI and FBG baseline was less obvious in some cases as 6 participant students exhibited impaired fasting glucose (IFG) without any apparent risk factor. Despite postprandial dysregulation, this may be caused by compensatory processes in early insulin resistance stages, keeping fasting glucose within normal levels. Such results highlight the possible drawbacks of fasting glucose by itself in spotting participants vulnerable to RH or IGT despite normal fasting blood glucose. This implies that regular screenings, depending only on fasting readings, can miss early postprandial dysglycaemia. Moreover, a subset of patients had reactive hypoglycemia, defined by a notable decrease in glucose levels following a glucose load, suggesting aberrant insulin dynamics or increased insulin sensitivity. It also raises the point that glucose homeostasis might come before or happen separately from obesity or clear insulin resistance, indicating early β-cell malfunction, heightened insulin sensitivity, or genetic abnormalities in control of glucose metabolism not yet clinically apparent [23].
Comparing between genders, it was observed that Females might show more insulin sensitivity than males, as seen in the above study, in early adulthood, hence influencing different RH or IGT prospective [24].
Conclusion
The current study emphasizes a notable positive relationship between BMI and 2-hour post-load FBG, therefore stressing the connection between higher adiposity and reduced glucose tolerance. Although BMI had little correlation with fasting glucose levels, its relationship to postprandial glucose responses seemed stronger, implying that postprandial measurements might be more sensitive indicators of metabolic risk in overweight persons. The role of BMI in reactive hypoglycemia is still unclear and needs more investigation. Especially in those with high BMI, the results indicate that 2-hr FBG values should be included in risk classification for glucose metabolism diseases.
Limitations: Above study has to be acknowledged with several limitations:
Sample Size and Demographics: Size and demographic homogeneity (e.g., age, race, or gender distribution) may cause the sample to be unrepresentative of the larger population.
Causal inference is limited by the cross-sectional design of the study.
Longitudinal data would better show directionality between changes in BMI and control of glucose.
No insulin measurements: It limit understanding of insulin resistance or hyperinsulinemia, which are fundamental to both IGT and RH.
BMI usage: Using BMI by itself could be misleading as it doesn't differentiate between fat mass and lean mass or consider fat distribution.
Limited Dietary and Lifestyle Data: All of which could influence glucose metabolism, dietary consumption, physical activity, and medication use were not controlled.
Recommendations:
- Future research should incorporate insulin assays—fasting and post-load—to more accurately evaluate insulin sensitivity and secretion patterns.
- Extra anthropometric indices are used: Waist-to-hip ratio or waist circumference could offer more knowledge about metabolic risk than BMI by itself.
- Longitudinal Studies: Prospective cohort studies are required to evaluate the transition from RH or IGT to overt diabetes and how BMI affects this path.
- Even if fasting glucose seems normal, doctors should think about including a 2-hour OGTT into screening for those with high BMI.
- Research on RH across different BMI ranges and demographic groups could assist understand its genesis and clinical relevance.
- Increase glucose tolerance testing in young adults regardless of BMI or risk factor profile, particularly if symptomatic (e.g., lethargy, post-meal dizziness).
- Follow-up research is required to see whether these early anomalies forecast future metabolic diseases such as type 2 DM or metabolic syndrome.
- Future studies ought to include insulin and C-peptide levels to assess pancreatic beta-cell activity and insulin dynamics.
- Need to investigate possible genetic predispositions to RH or IGT in this population lacking standard risk factors.
Awareness should be raised on balanced diet and glycaemic fluctuation even among healthy-weight people, perhaps employing continuous glucose monitoring (CGM) for chosen individuals
References
- Siperstein M. The glucose tolerance test: a pitfall in the diagnosis of diabetes mellitus. Advances in internal medicine, 1975; 20: 297-323.
- Bang I, Blutzucker D. Verlag Von JF Bergmann. In: Wiesbaden, 1913.
- De Nobel E, Van't Laar A. The size of the loading dose as an important determinant of the results of the oral glucose tolerance test: a study in subjects with slightly impaired glucose tolerance. Diabetes, 1978; 27(1): 42-48.
- Wingerd J, Duffy TJ. Oral contraceptive use and other factors in the standard glucose tolerance test. Diabetes, 1977; 26(11): 1024-1033.
- Jamieson EL, Dimeski G, Flatman R, Hickman PE, Jones GRD, Marley JV, et al. Oral glucose tolerance test to diagnose gestational diabetes mellitus: impact of variations in specimen handling. Clinical biochemistry, 2023; 115: 33-48.
- Jagannathan R, Neves JS, Dorcely B, Chung ST, Tamura K, Rhee M, et al. The oral glucose tolerance test: 100 years later. Diabetes, Metabolic Syndrome and Obesity, 2020; 3787-3805.
- Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Reviews Endocrinology, 2018; 14(2): 88-98. https://doi.org/10.1038/nrendo.2017.151
- Rao SS, Disraeli P, McGregor T. Impaired glucose tolerance and impaired fasting glucose. Am Fam Physician, 2004; 69(8): 1961-1968.
- Ramlo-Halsted BA, Edelman SV. The natural history of type 2 diabetes: implications for clinical practice. Primary Care: Clinics in Office Practice, 1999; 26(4): 771-790.
- Pietropaolo M, Le Roith D. Pathogenesis of diabetes: our current understanding. Clinical cornerstone, 2001; 4(2), 1-16.
- World Health Organization. Regional Office for the Western P. The Asia-Pacific perspective : redefining obesity and its treatment. Sydney : Health Communications Australia, 2000.
- https://iris.who.int/handle/10665/206936
- Phillips PJ. Oral glucose tolerance testing. Aust Fam Physician, 2012; 41(6), 391-393.
- Malik A, Sharif A, Zubair HM, Akhtar B, Mobashar A. In vitro, in silico, and in vivo studies of Cardamine hirsuta Linn as a potential antidiabetic agent in a rat model. ACS Omega, 2023; 8(25): 22623-22636.
- Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, et al. Full accounting of diabetes and pre-diabetes in the US population in 1988–1994 and 2005–2006. Diabetes care, 2009; 32(2): 287-294.
- Unwin N, Shaw J, Zimmet P, Alberti K. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabetic medicine, 2002; 19(9).
- Alqahtani N, Khan WAG, Alhumaidi MH, Ahmed YAAR. Use of glycated hemoglobin in the diagnosis of diabetes mellitus and pre-diabetes and role of fasting plasma glucose, oral glucose tolerance test. International journal of preventive medicine, 2013; 4(9): 1025.
- Davidson MB, Schriger DL, Peters AL, Lorber B. Relationship between fasting plasma glucose and glycosylated hemoglobin: potential for false-positive diagnoses of type 2 diabetes using new diagnostic criteria. Jama, 1999; 281(13): 1203-1210.
- Wang X, Zhao X, Zhou R, Gu Y, Zhu X, Tang Z, et al. Delay in glucose peak time during the oral glucose tolerance test as an indicator of insulin resistance and insulin secretion in type 2 diabetes patients. J Diabetes Investig, 2018; 9(6): 1288-1295. https://doi.org/10.1111/jdi.12834.
- Alberti KG. The clinical implications of impaired glucose tolerance. Diabet Med, 1996; 13(11): 927-937. https://doi.org/10.1002/(sici)1096-9136(199611)13:11
- Kawazu S. Definition and diagnosis of IFG, IGT and borderline type]. Nihon Rinsho, 2005; 63 Suppl 2, 43-48.
- Khuon D, Rupasinghe D, Saphonn V, Kwong TS, Widhani A, Chaiwarith R, et al. BMI as a predictor of high fasting blood glucose among people living with HIV in the Asia-Pacific region. HIV Med, 2023; 24(2): 139-152. https://doi.org/10.1111/hiv.13351
- Martyn JA, Kaneki M, Yasuhara S. Obesity-induced insulin resistance and hyperglycemia: etiologic factors and molecular mechanisms. Anesthesiology, 2008; 109(1): 137-148. https://doi.org/10.1097/ALN.0b013e3181799d45
- Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med, 2009; 6 Suppl 1(Suppl 1), 60-75. https://doi.org/10.1016/j.genm.2009.02.002.

