Pancreatic Neuroendocrine Tumor Recurrence after Tirzepatide Use
Nathan Do BA1,*, Priyanka Venkatesh2, Alok Tripathi2 and Anup Kasi3
1The University of Kansas School of Medicine, Kansas City, USA
2Department of Internal Medicine, The University of Kansas Medical Center, Kansas City, USA
3Department of Clinical Oncology, The University of Kansas Medical Center, Westwood, USA
Received Date: 21/04/2025; Published Date: 04/06/2025
*Corresponding author: Nathan Do BA, The University of Kansas School of Medicine, Kansas City, USA
DOI: 10.46998/IJCMCR.2025.52.001285
Keywords: Tirzepatide; Recurrence; Neuroendocrine Tumors; Pancreatic Neoplasms
Introduction
Pancreatic neuroendocrine tumors (PNETs), or islet cell tumors, arise from pancreatic endocrine tissue [1]. While most fall within non-functional PNETs, about 25% to 50% are functional PNETs [1]. Following pancreatectomy, drugs such as tirzepatide (TRZD) may be prescribed to address endocrine insufficiency. Tirzepatide is a dual agonist for glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) which stimulate insulin secretion [2]. While TRZD can enhance insulin secretion, it carries risks, including pancreatitis and a black box warning for C-cell tumors, particularly in patients with a history of medullary thyroid cancer or multiple neuroendocrine neoplasia type 2 (MEN2) [2]. Additionally, concerns have arisen about its potential role in promoting PNETs or their recurrence. Although some incretin therapies have shown anti-cancer effects, retrospective studies indicate that incretin mimetics may be associated with pancreatic alpha cell hyperplasia and glucagon-producing neuroendocrine tumors [3].
This case discusses a patient who developed diabetes after a distal pancreatectomy due to pancreatic endocrine insufficiency. Initially treated with insulin and metformin, she discontinued metformin due to intolerance and later added TRZD to manage her diabetes and support weight loss. However, two months following initiation of TRZD therapy, she experienced recurrence of the PNET. This highlights the need for careful consideration of TRZD use in patients with a history of PNETs.
Case Presentation
We present a 57-year-old patient with a past medical history of gestational diabetes mellitus, hyperlipidemia, and angioedema, who initially presented with one year of abdominal pain. Imaging at another hospital revealed a pancreatic mass, leading to referral to our hospital. Magnetic resonance imaging (MRI) showed an 8.5 cm x 8.5 cm complex cystic pancreatic body and tail region mass. The patient underwent exploratory laparotomy, distal pancreatectomy, splenectomy, and cholecystectomy. Pathology identified a low-grade well-differentiated PNET. The pathological stage was pT2N0M0. Further staging workup revealed a clinical stage IB PNET, and the patient was treated with curative intent. Chromogranin A, Pancreatic Polypeptide (PPP), and urinary 5-hydroxyindole-acetic acid (5-HIAA) were within normal limits and the patient was placed on active surveillance. The patient was noted to be hyperglycemic due to endocrine pancreatic insufficiency and was started on metformin. She did not tolerate metformin and was subsequently started on insulin. Eight years after her initial surgery, the patient was noted to have an enlarging mild right anterior diaphragmatic and paracaval lymph nodes on Computed Tomography (CT) of the abdomen/pelvis. She then underwent a dotatate neuroendocrine Positron Emission Tomography (PET) scan which showed two discrete foci of intense tracer uptake in the upper retroperitoneum at midline superior to the distal pancreatectomy site, without distinct CT correlate, compatible with somatostatin receptor-positive neuroendocrine tumor implants, as seen in Figure 1. There also was tracer avid anterior diaphragmatic and middle diaphragmatic nodal metastatic disease. The patient reported that she had started TRZD one month prior to this visit. Following a discussion at our tumor board, the patient was referred to interventional radiology for a CT-guided biopsy of the anterior diaphragmatic lymph node. Pathology was consistent with metastatic well-differentiated neuroendocrine tumor. Ki-67 was 4% which was consistent with World Health Organization (WHO) Grade G2. Chromogranin, urine 5-HIAA, and PPP were rechecked and noted to be within normal limits. Tirzepatide was stopped and the patient was started on octreotide long acting repeatable (LAR) with an initial dose of 10 mg and then increased to 20 mg for subsequent injections. PET scan was done after three injections, which showed redemonstration of the radiotracer avid lymph nodes but decreased in size compared to prior imaging. The patient remains on octreotide monthly and will have imaging prior to cycle 7.
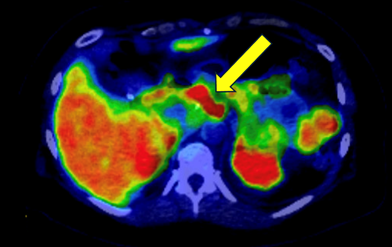
Figure 1: Positron emission tomography-computed tomography (PET-CT) scan indicating tracer activity uptake in the upper retroperitoneum at midline superior to the distal pancreatectomy site.
Discussion
PNETs are rare neoplasms that exhibit varying degrees of malignant potential.3 This case highlights a recurrence of PNET in a patient who had been in remission for eight years prior to the initiation of TRZD, raising concerns about the potential role of TRZD in tumor recurrence.
Mechanistic Insight
Tirzepatide and Tumor Biology: For pancreatic beta cells, the insulin secretion pathway from TRZD-use includes glucagon-like peptide-1 receptor (GLP-1R) and glucose-dependent insulinotropic polypeptide receptor (GIP-R) activation via the adenylyl cyclase-cAMP-protein kinase A pathway that activates glycolytic and mitochondrial metabolism of glucose. The increase in adenosine triphosphate (ATP) leads to the closure of plasma membrane K+ channels which cause beta-cell depolarization– the opening of voltage gated Ca+ and immediate influx of Ca+ into the cell. The increase in cytosolic calcium leads to calcium release from the endoplasmic reticulum and eventually the release of insulin into the bloodstream. In this pathway lies a potential oncogenic property: the activation of insulin gene transcription [4].
The activation of protein kinase A leads to the synthesis of insulin, insulin-like growth factors 1 and 2 (IGF-1 and –2), and insulin-like growth factor binding protein 3 (IGF-BP3). Such products can activate key growth pathways such as PI3K/Akt/mTOR, Ras-Raf-ERK, and JAK-STAT signaling in target cells, ultimately leading to cancer and its potential recurrence by supporting cellular proliferation and growth, aberrant cancer metabolism, inhibition of cell death, invasion, and metastasis in these tissues, as seen in Figure 2 [4].
Although PNETs are generally considered to have a good overall prognosis after resection, well-differentiated nonfunctional-PNETs (grade 1 and 2) have a reported recurrence rate of 17% after resection [5]. In this case, the patient developed metastatic recurrence of a well-differentiated PNET soon after starting TRZD after being in remission for eight years post-surgery, a time when recurrence rates typically are very low. This suggests a close temporal association with RZD initiation in stimulating tumor growth.
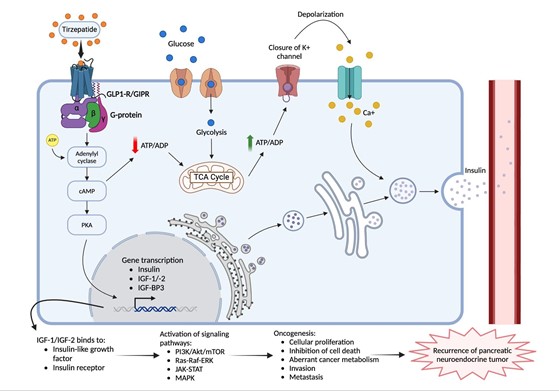
Figure 2: Tirzepatide (TZRD) pathway in pancreatic beta cells indicating insulin release into bloodstream and process of recurrence of pancreatic neuroendocrine tumor via growth signaling pathways.
Clinical Implications: Caution in High-Risk Patients: The use of TRZD and other incretin-based therapies in patients with a history of previously confirmed neuroendocrine tumors warrants caution. Preclinical evidence suggests that incretin mimetics may promote pancreatic alpha cell hyperplasia and contribute to glucagon-producing neuroendocrine tumors.3 This case raises concerns that such therapies could reactivate dormant tumor cells or enhance the growth of indolent metastatic disease.
In patients with a history of PNETs, clinicians should carefully weigh the risks and benefits of the use of TRZD for glycemic control against the potential risks of tumor stimulation. Alternatives, such as insulin or non-incretin-based therapies, should be considered, especially in patients with a previous history of neuroendocrine tumors.
Limitations and Research Gaps: A major limitation in this case report is the inability to perform GLP-1R and GIP-R staining on the tissue of recurrent tumors due to institutional restrictions. Such a stain could have provided critical insights into receptor-mediated tumorigenesis. Additionally, the current knowledge about the mechanism of incretin-based therapies on neuroendocrine tumor behavior is confined to retrospective studies and preclinical models [3,6].
Future prospective studies are needed to assess the long-term safety of incretin-based therapies in patients with a history of neuroendocrine tumors. In addition, mechanistic studies will be needed to further delineate the role of GLP-1 and GIP pathways that could contribute to tumor progression and recurrences. Better clinical access to receptor staining for targeted tumor analysis will yield important information on receptor-mediated oncogenic mechanisms, which will enable more precise and personalized therapeutic interventions.
Conclusion
This case report illustrates the potential role of GLP-1 and GIP agonists in the malignant transformation of the pancreas. While TRZD offers significant benefits for diabetes management, its potential role in promoting tumor recurrence requires further investigation. Until more robust evidence is available, clinicians should exercise caution when considering incretin-based therapies for patients at risk for neuroendocrine tumors.
The authors confirm that informed consent has been obtained from the patient for publication of this case report. The manuscript has not been published or submitted elsewhere, and all relevant ethical guidelines were followed. No conflicts of interest or financial support were involved in the preparation of this report.
I, Nathan Do, confirm that I have carefully reviewed the material and endorse its contents.
I, Priyanka Venkatesh, confirm that I have carefully reviewed the material and endorse its contents.
I, Dr. Alok Tripathi, confirm that I have carefully reviewed the material and endorse its contents.
I, Dr. Anup Kasi, confirm that I have carefully reviewed the material and endorse its contents.
References
- Ro C, Chai W, Yu V, Yu R. Pancreatic neuroendocrine tumors: biology, diagnosis, and treatment. Chin J Journal of Cancer, 2013; 32(6): 312-324.
- Farzam K, Patel P. Tirzepatide. 2024.
- Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes, 2013; 62(7): 2595–2604.
- Samuel SM, Varghese E, Kubatka P, Busselberg D. Tirzepatide– Friend or Foe in Diabetic Cancer Patients? Biomolecules, 2022; 12(21): 1580.
- Tan QQ, Wang X, Yang L, et al. Analysis of recurrence after resection of well-differentiated non-functioning pancreatic neuroendocrine tumors. Medicine, 2020; 99(24): e20324.
- Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab, 2013; 17(6): 819-837.

