Impact of COVID-19 Vaccination on Cancer Patients in Dubai Hospital, DAHC. A Single Centre Experience from the COVID-19 Pandemic
Syed Hammad Tirmmazy D, Muhammad Farooq Latif D, Dalia Elshourbagy D and Mohamed Omara Ibrahim Hussien*
1Department of Oncology, Dubai Hospital, UAE
2Associate Professor of Oncology, Mohammed Bin Rashid University of medicine and health sciences (MBRU), UAE
3Assistant Professor of Oncology, Mohammed Bin Rashid University of medicine and health sciences (MBRU), UAE
Received Date: 10/04/2025; Published Date: 27/05/2025
*Corresponding author: Dr. Mohamed Omara Ibrahim Hussien, Department of Oncology, Dubai Hospital, Al Khaleej st, Al Baraha, Dubai, UAE - Clinical Assistant Professor of Oncology, Mohammed Bin Rashid University of medicine and health sciences (MBRU), UAE
Abstract
Aims: Cancer patients are at increased risk of infections due to their immunocompromised state [1]. During the Covid-19 pandemic, early reports confirmed that people with cancer were at high risk for severe complications from COVID-19 infection [4-6]. We conducted this study to assess the clinical effectiveness of Covid-19 vaccine in cancer patients.
Methods: Data was collected from the hospital electronic medical record system for all patients with solid and haematological malignancies who received at least one dose of covid-19 vaccination from January 2021 to October 2022.
Results: A total of 2923 patients had received at least one dose of approved covid-19 vaccination. Median age of the patients was 56 (12-93) years. Majority of the patients (60 %) were female and had a diagnosis of solid tumours (94%). A total of 1322 cancer patients including 819 (62%) female and 507 (38%) male patients have been diagnosed with Covid-19 infection.Overall 137 (10%) covid positive patients died including 73 male (median age 70 years) and 64 female patients (median age 63 years). Higher rate of covid 19 infection related mortality was observed in unvaccinated patients (n=116, 17%) as compared to the vaccinated cohort (n=21, 3%). Median age in the deceased patients was higher (median age 66 years) as compared to alive patients with median age 57 years.
Conclusion: Overall mortality was lower in vaccinated patients who acquired covid-19 infection. Systemic anticancer therapy can be administered safely with strict adherence to infection control measures in vaccinated patients.
Introduction
Cancer patients are at increased risk of infections due to their immunocompromised state [1]. Systemic treatment including chemotherapy, and other targeted treatment further increases the risk of infection [2]. The World Health Organization (WHO) declared the novel coronavirus (COVID-19) outbreak as global pandemic in March 2020. Over 600 million people have been infected with unfortunately over 6 million confirmed deaths globally [3]. During the Covid-19 pandemic there were concerns of severe morbidity and mortality from acquiring this infection and unfortunately early reports confirmed that people with cancer were at high risk for severe complications from COVID-19 [4-6].
Disruption in routine health care services were also leading to delay in cancer screening, diagnosis, treatment and surveillance. In addition, concerns were raised about patient recruitment in clinical trials [10]. Several clinical trials were initiated globally to develop and evaluate the safety and efficacy of different covid-19 vaccines. First Covid-19 vaccine was approved by FDA for emergency use on August 23, 2021 [8]. Since then, several covid-19 vaccines have been approved. The guidelines from major health organisations included cancer patients amongst the high-risk population and recommended vaccination of cancer patients as a priority [7,8].
Dubai Academic Health Corporation (DAHC) was one of the first organisations in the region who implemented these recommendations very early on during the pandemic [9]. Although anti-infective vaccinations have been shown to be safe and effective in cancer patients, reduced protective effects may occur in patients undergoing certain immunosuppressive systemic anti-cancer treatments [11,12].
We conducted this study to assess the clinical effectiveness of Covid-19 vaccine in preventing covid-19 related morbidity and mortality in cancer patients being treated at DAHC.
Methods
This is a retrospective study where we reviewed the electronic medical records of cancer patients treated in Dubai Academic Health Corporation during covid 19 pandemic from January 2021 to October 2022. The study was approved by the Dubai scientific research ethics committee. Eligibility criteria included all patients with solid tumours and haematological malignancies who received at least one dose of covid 19 vaccination either Pfizer Bio-NTech or Sinopharm during the study period.
Eligible patients were identified through the hospital cancer registry and data were extracted using Epic SlicerDicer self-reporting tool. Data regarding patients’ demographics, type of cancer, type of treatment, type and number of covid-19 vaccination, covid-19 PCR results, covid-19 related morbidity and mortality was collected retrospectively. After initial screening, patients were divided into 2 main categories namely partially vaccinated (one dose of vaccination) and fully vaccinated (2 doses of vaccination with or without a booster dose). Frequency of the use of approved vaccines (Pfizer Bio-NTech and Sinopharm) was assessed in each group.
Then the total number of Covid 19 positive cases were identified during the study period. The incidence of covid 19 infections and mortality was calculated and cross compared amongst vaccinated and unvaccinated cohorts. Impact of systemic anticancer therapy administration within 6 weeks of Covid 19 PCR positivity and mortality was then assessed. Data was analysed using Microsoft Excel version 1808.
Results
After the emergency approval of covid-19 vaccination, the first cancer patient in DAHC received the first dose of vaccination on 23rd December 2020. Pfizer Bio-NTech and Sinophram were the approved vaccinations. (Figure 1) A total of 2923 patients had received at least one dose of approved covid-19 vaccination till 1st October 2022 including Pfizer Bio-NTech (n=2672, 91%) and Sinopharm (n=251, 9%). 102 patients received both vaccines. Out of 2871 fully vaccinated patients who received 2 doses of vaccines, 2630 (92%) received Pfizer and 241 (8%) patients received Sinopharm vaccines. 868 (30%) fully vaccinated patients also received 1 booster dose. Ninety-eight patients received both Pfizer and Sinopharm vaccines.
Majority of the patients received covid 19 vaccination during the year 2021 (n=2739, 94%) including Pfizer Bio-NTech (1st dose n=2488, 91%, 2nd dose n=2404, 91%) and Sinopharm (1st dose n=251, 9%, 2nd dose n=241, 9%). (Figure 2) Pfizer Bio-NTech was the only vaccine used in the year 2022 (1st dose n=184, 2nd dosen=226). Median age of the patients was 56 (12-93) years. Majority of the patients (60%) were female, above the age of 18 years (92%) and had diagnosis of solid tumours (94%).
On 4th April 2020, 1st case of Covid-19 was reported in a cancer patient in DHA. A total of 1322 cancer patients including 819 (62%) female and 507 (38%) male patients have been diagnosed with Covid-19 infection till the end of this study with a median age of 58 years. (Figure 3) 648 (49%) covid positive patients received Pfizer Bio-NTech (n=619, 96%) or Sinopharm vaccination (n=29, 4%) and were reported covid-19 positive either before or after the vaccination. 674 (51%) unvaccinated patients developed covid 19 infection. Overall, 137 (10%) covid positive patients died including 73 male (median age 70 years) and 64 female patients (median age 63 years). Higher rate of covid 19 infection related mortality was observed in unvaccinated patients (n=116, 17%) as compared to the vaccinated cohort (n=21, 3%).
In the unvaccinated covid positive group, 116 (17%) patients died. Median age in the deceased patients was higher (median age 66 years, male 70 years, female 62 years) as compared to alive patients with median age 57 years.
Out of the 21 patients who died in the vaccinated cohort, there were 11 female and 10 male patients. Deceased patients were 10 years older with median age of 67 years (male 69 years, female 65 years) as compared to the rest of the patients in this group with median age of 57 years (male 62 years, female 53 years) (Figure 4).
Seventeen (81%) patients had diagnosis of solid cancers and four (19%) patients had haematological malignancies. Three (14%) patients received systemic anti-cancer therapy including Abiraterone (n=1), Fluorouracil/Irinotecan (n=1) and Immunotherapy (n=1) within 6 weeks prior to Covid 19 infection related death. None of these patients developed anti-cancer treatment induced neutropenia.
Our study concluded that covid 19 infection related mortality was higher in elderly and unvaccinated cancer patients. Systemic anticancer therapy can be administered safely with strict adherence to infection control measures in vaccinated patients without any increased risk of covid 19 infection related complications or death.
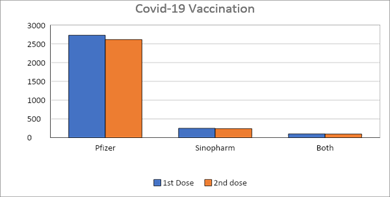
Figure 1: Types of approved covid-19 vaccines administered in the study population.
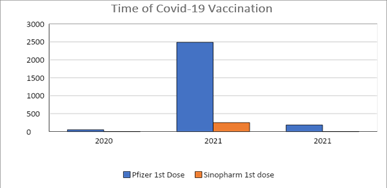
Figure 2: Timeline of Covid-19 vaccination in DAHC.
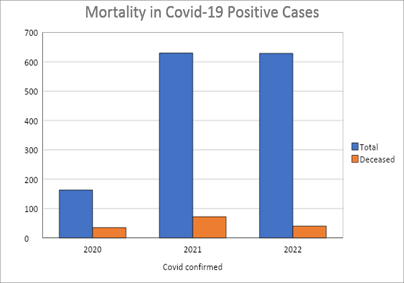
Figure 3: Overall mortality in covid-19 infected patients.
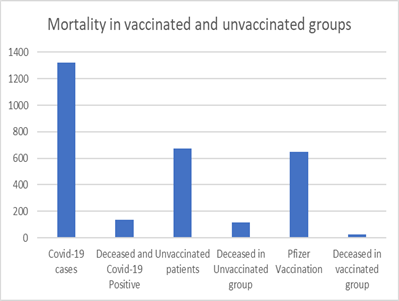
Figure 4: Mortality in the covid-19 vaccinated and unvaccinated patients.
Discussion
Cancer patients are at increased risk of infections due to their immunocompromised state. Contributing factors include patients’ pre-morbid health, performance status, type of malignancy and treatment modalities [1]. Systemic treatment including chemotherapy, and other targeted treatment can further increase the risk of infection [2]. The World Health Organization (WHO) declared the novel coronavirus (COVID-19) outbreak as global pandemic in March 2020. Over 600 million people have been infected with unfortunately over 6 million confirmed deaths globally. Public health measures were introduced to reduce the risk of viral transmission [3]. Cancer patients were reported to have increased risk of severe covid-19 infection, mechanical ventilation and ICU admission. Furthermore, patients who had received chemotherapy or undergone surgery in the 30 days before contracting COVID-19 were found to have a higher risk of severe complications than patients who had not been treated with chemotherapy or surgery. Other studies reported that an anti-cancer treatment within 14 days of COVID-19 infection was a risk factor for acute respiratory distress syndrome, septic shock, and acute myocardial infarction [4]. Kuderer et al reported that 30-day all-cause mortality was high among cancer patients with covid-19 infection [5].
There were also concerns of significant delay in cancer screening, diagnosis, treatment and surveillance ultimately leading to increased risk of cancer-related morbidity and mortality [9]. In general, anti-infective vaccinations have been shown to be safe and effective in cancer patients. However, reduced protective effects may occur in patients undergoing certain systemic anti-cancer treatments. Ideally vaccination should be administered before the commencement of systemic therapy, although vaccination during the treatment may be implemented [10,11]. Several clinical trials were initiated globally to develop and evaluate the safety and efficacy of different covid-19 vaccines.
Polack FP et al reported results of multinational, placebo-controlled, observer-blinded, pivotal efficacy trial, evaluating the Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine which conferred 95% protection against Covid-19 in persons 16 years of age or older [13]. FDA authorised emergency use of Pfizer-BioNTech COVID-19 vaccine on August 23, 2021 [8]. Since then, several covid-19 vaccines have been approved including, Moderna COVID-19 Vaccines, Janssen COVID-19 Vaccine and Novavax COVID-19 Vaccine. Major health organisations across the world recommended emergency use of covid-19 vaccination. However initially there was a lack of safety and efficacy data for covid-19 vaccination in cancer patients as they were generally excluded from these trials.
It was noted that the rate of seroconversion was significantly lower following only one dose of an mRNA vaccine hence the importance of vaccination completion and booster was advised for cancer patients [12]. VOICE and CAPTURE studies reported significantly inferior antibody response after only one dose of the vaccine, However, after full vaccination above 80% patients with cancer developed neutralising antibodies [13,14]. In a real-world study of patients with cancer, only 29% of patients had an antibody response after the first vaccine dose, compared with 84% of controls (P< 0.001). However, after the second dose seroconversion rate was 86% [15].
There remains a subgroup of cancer patients with impaired immune response following anti-SARS-CoV-2 vaccination especially haematological malignancies compared with patients with solid tumours [16]. In particular a lower seropositivity rate following vaccination was reported for patients treated with cytotoxic chemotherapy compared with other cancer patients [17].
In practice it is important to assess the clinical effectiveness of vaccination such as rates of infection, COVID-19 disease severity and mortality. Embi et al. reported that mRNA vaccine effectiveness against COVID-19–associated hospitalisation was clinically significant among immunocompromised patients but lower than immunocompetent patients [18]. Another study concluded that vaccinated patients with cancer achieved clinical effectiveness of 57% for patients on chemotherapy, 76% for those on endocrine therapy and 85% for those not receiving systemic therapy [19].
Hence guidelines from major health organisations included cancer patients amongst the high-risk population and recommended vaccination of cancer patients as a priority [6,7]. DAHC was one of the first organisations in the region who implemented these recommendations very early on during the pandemic [8].
We recommended that the majority of our patients undergo Covid-19 vaccination after thorough counselling and reassurance. Majority of the patients (91%) received Pfizer Bio-NTech vaccine at DAHC, and the vast majority completed at least 2 doses of the approved vaccine. We also recommended Booster vaccine to our patients.
Our study concluded that there was significant covid-19 infection-related mortality among unvaccinated older cancer patients as was shown in the initial reports during the pandemic [4,5]. Earlier studies had shown lower seroconversion following one dose of covid-19 vaccine which improved after the 2nd dose, however clinical significance of vaccination was not known.
We noticed significant differences in all-cause mortality in covid-19 infected patients in the vaccinated and unvaccinated group. There was higher mortality amongst unvaccinated groups. Covid-19 vaccine reduced the risk of mortality amongst covid-19 infected patients. However, the risk of acquiring Covid-19 infection was similar between the two groups.
Conclusion
This is the first real world study from the Gulf region reporting the outcome of covid-19 vaccination in cancer patients. It confirms the clinical effectiveness of vaccination in this high-risk population. Overall mortality was lower in vaccinated patients who acquired covid-19 infection. Systemic anticancer therapy can be administered safely with strict adherence to infection control measures in vaccinated patients without any increased risk of covid 19 infection related complications or death.
Limitations of our study: The study provides insights into COVID-19 vaccine effectiveness in cancer patients but has limitations. It relies on retrospective data from electronic records, potentially introducing bias, and lacks a control group for comparison, limiting causal inference. Conducted in a single hospital, its generalizability is constrained. Additionally, the study does not account for other factors such as comorbidities or cancer stage, which could impact COVID-19 outcomes. While suggesting a potential benefit of vaccination in reducing mortality, further research with robust methodologies and larger, more diverse cohorts is warranted for validation and broader applicability.
References
- Zembower Epidemiology of infections in cancer patients. In: Cancer treatment and research. Cham: Springer International Publishing, 2014; 161: 43-89.
- Bow EJ. Infection risk and cancer chemotherapy: The impact of the chemotherapeutic regimen in patients with lymphoma and solid tissue Journal of antimicrobial chemotherapy, 1998; 41 Suppl D (suppl 4): 1-5. doi: 10.1093/jac/41.suppl_4.1.
- World health organization, 2019.
- Al-Quteimat OM, Amer The impact of the COVID-19 pandemic on cancer patients. American Journal of Clinical Oncology, 2020; 43(6): 452-455. doi: 10.1097/COC.0000000000000712.
- Choueiri TK, Shyr Y, Grivas P, et Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. The Lancet, 2020; 395(10241): 1907-1918. https://dx.doi.org/10.1016/S0140-6736(20)31187-9. doi: 10.1016/S0140-6736(20)31187-9.
- Fong D, Rauch S, Petter C, Haspinger E, Alber M, Mitterer M. Infection rate and clinical management of cancer patients during the COVID-19 pandemic: Experience from a tertiary care hospital in northern italy. ESMO open, 2020; 5(3): e000810. http://dx.doi.org/10.1136/esmoopen-2020-000810. doi: 1136/esmoopen-2020-000810.
- WHO COVID-19 advice for the public: Getting vaccinated.
- FDA covid-19 vaccines, 2022.
- Boughey JC, Snyder RA, Kantor O, et Impact of the COVID-19 pandemic on cancer clinical trials. Ann Surg Oncol, 2021; 28(12): 7311-7316. doi: 10.1245/s10434-021-10406-2.
- Ward EM, Flowers CR, Gansler T, Omer SB, Bednarczyk RA. The importance of immunization in cancer prevention, treatment, and survivorship. CA: a cancer journal for clinicians, 2017; 67(5): 398-410. doi: 10.3322/caac.21407.
- Rieger CT, Liss B, Mellinghoff S, et Anti-infective vaccination strategies in patients with hematologic malignancies or solid tumors—Guideline of the infectious diseases working party (AGIHO) of the german society for hematology and medical oncology (DGHO). Annals of oncology, 2018; 29(6): 1354-1365. https://dx.doi.org/10.1093/annonc/mdy117. doi: 10.1093/annonc/mdy117.
- Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. The lancet oncology, 2021; 22(6): 765-778. https://dx.doi.org/10.1016/S1470-2045(21)00213-8. doi: 1016/S1470-2045(21)00213-8.
- Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: A prospective, multicentre, non-inferiority trial. The lancet oncology, 2021; 22(12): 1681-1691. https://dx.doi.org/10.1016/S1470-2045(21)00574-X. doi: 10.1016/S1470-2045(21)00574-
- Shepherd STC, Fendler A, Au L, et al. 1557O adaptive immunity to SARS-CoV-2 infection and vaccination in cancer patients: The CAPTURE Annals of oncology, 2021; 32: S1129. https://dx.doi.org/10.1016/j.annonc.2021.08.1550. doi: 10.1016/j.annonc.2021.08.1550.
- Ben-Aharon I, Waldhorn I, Holland R, Peer A, Halberthal M, Goshen - Lago 1559O efficacy and toxicity of BNT162b2 vaccine in cancer patients. Annals of oncology, 2021; 32: S1130. https://dx.doi.org/10.1016/j.annonc.2021.08.1552. doi: 10.1016/j.annonc.2021.08.1552.
- Ehmsen S, Asmussen A, Jeppesen SS, et Antibody and t cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer cell, 2021; 39(8): 1034-1036. https://dx.doi.org/10.1016/j.ccell.2021.07.016. doi: 10.1016/j.ccell.2021.07.016.
- Thakkar A, Gonzalez-Lugo JD, Goradia N, et Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer cell, 2021; 39(8): 1081-1090.e2. https://dx.doi.org/10.1016/j.ccell.2021.06.002. doi: 10.1016/j.ccell.2021.06.002.
- Embi PJ, Levy ME, Naleway AL, et Effectiveness of two-dose vaccination with mRNA COVID-19 vaccines against COVID-19–associated hospitalizations among immunocompromised adults—Nine states, January–September 2021. American Journal of Transplantation, 2022; 22(1): 306-314. https://onlinelibrary.wiley.com/doi/abs/10.1111/ajt.16641. doi: 10.1111/ajt.16641.
- Wu JT, La J, Branch-Elliman W, et 1562MO effectiveness of COVID-19 vaccination in cancer patients: A nationwide veteran’s affairs study. Annals of oncology, 2021; 32: S1131. https://dx.doi.org/10.1016/j.annonc.2021.08.1555. doi: 10.1016/j.annonc.2021.08.1555.

