Sepsis-Induced Cardiac Arrest Secondary to Multidrug-Resistant Urinary Tract Infection in Obstructive Uropathy: A Rare Case Report and Literature Review
Abshiro H Mayow1,* and Samer Kholoki2
1George’s University School of Medicine, Grenada
2UChicago Medicine Advent Health La Grange Memorial Hospital, Chicago, USA
Received Date: 24/03/2025; Published Date: 25/04/2025
*Corresponding author: Abshiro H Mayow, George’s University School of Medicine, Grenada
Abstract
Urinary Tract Infections (UTIs) represent a common yet significant cause of sepsis among hospitalized patients, ranging from uncomplicated infections managed effectively in outpatient settings to complicated UTIs that can rapidly escalate into life-threatening urosepsis. Complicated UTIs are frequently associated with obstructive uropathy, particularly in elderly patients with comorbidities such as diabetes mellitus, chronic kidney disease, and structural abnormalities like nephrolithiasis. Severe infections, notably those caused by resistant pathogens such as extended-spectrum beta-lactamase (ESBL)-)-producing Escherichia coli poses substantial diagnostic and therapeutic challenges. In severe cases, the systemic inflammatory response can trigger cardiac complications, including atrial fibrillation (AFib) and Sepsis-Induced Cardiac Arrest (SICA), a devastating event characterized predominantly by non-shockable rhythms, such as Pulseless Electrical Activity (PEA). Despite its clinical importance, the pathophysiology of SICA in the context of complicated UTIs remains underreported and poorly understood, representing a critical gap in current knowledge.
This case report investigates a rare case of a 63-year-old nursing home resident with a significant history of recurrent obstructive uropathy, complicated UTIs, stage 3 chronic kidney disease, diabetes mellitus, and chronic anemia who developed severe urosepsis due to ESBL-producing E. coli infection. Upon hospitalization, the patient's condition rapidly progressed to septic shock complicated by new-onset AFib with rapid ventricular response, ultimately culminating in cardiac arrest presenting as PEA. Successful resuscitation was accomplished through immediate advanced cardiovascular life support (ACLS), aggressive fluid resuscitation, rapid initiation of targeted antibiotic therapy with meropenem, and urgent urological intervention through bilateral ureteral stenting for definitive source control. The stabilization and eventual recovery of the patient, with discharge from the hospital later, were affected by multidisciplinary team management led by those in internal medicine, cardiology, nephrology, urology, critical care, and infectious diseases. Thus, this report highlights the lethal synergy between obstructive uropathy-associated sepsis and cardiac complications, indicating the need for heightened clinical suspicion and proactive cardiovascular monitoring in high-risk septic patients to improve outcomes.
Introduction
A urinary tract infection (UTI) is one of the most common global bacterial infections and is estimated to cause more than 400 million cases each year. UTIs are further classified as uncomplicated (i.e., affecting immunocompetent individuals with a typical urinary tract configuration) or complicated (when the infection is associated with risk factors such as old age, diabetes, obesity, prolonged catheter use, or obstructive uropathy) [1,2]. Escherichia coli accounts for most uncomplicated UTIs, which are usually treated on an outpatient basis [1]. Complicated UTIs, however, are more often due to multidrug-resistant pathogens (such as Klebsiella pneumoniae, Proteus mirabilis, and Enterococcus faecalis) due to biofilm formation and immunological compromise requiring differential antimicrobial management. A dangerous situation is created through indwelling catheters that facilitate infection, a 10% daily incidence of bacteriuria, and a 25% advancement to sepsis [3]. Obstructive uropathy, due to structural abnormalities, nephrolithiasis, or chronic inflammation, aggravates urinary stasis and bacterial growth, paving the way for urosepsis [4]. The effect of sepsis at the global level, which is calculated to affect 48.9 million individuals every year with >11 million deaths reported, makes it about 19% lucidity. Antimicrobial Resistance (AMR) is one of the reasons for the exacerbation of sepsis, particularly with the Horizontal Gene Transfer (HGT) of resistance determinants under antimicrobial pressure [5]. This highlights an urgent need for pathogen-specific biomarkers to inform precision management strategies to minimize the chances of improper empiric therapy and AMR dissemination.
Urosepsis, a type of sepsis due to a severe complication of UTIs, occurs when pathogens enter the bloodstream, triggering a cascade of inflammatory and immune responses. "Compared to high-income countries (HICs), low- and middle-income countries (LMICs) face a disproportionately heavier burden [6]. Various studies have observed variability at a significant regional level. From at least 11.9% in Oceania to 39.5% in Africa, mortality from sepsis shows the need for region-specific management strategies [7]. Sepsis disproportionately impacts individuals with chronic kidney disease (CKD), diabetes, and recurrent UTIs, as these conditions compromise immune function, delay bacterial clearance, and increase susceptibility to severe complications [8]. In its most severe form, sepsis progresses to septic shock, characterized by profound hemodynamic instability and multi-organ dysfunction. Approximately 30% of hospitalized patients with severe sepsis die, while septic shock mortality can exceed 50% [9].
In rare but catastrophic cases, sepsis progresses to sepsis-induced cardiac arrest (SICA), a phenomenon driven by profound myocardial depression, systemic vasodilation, impaired oxygen delivery, and an overwhelming inflammatory response [10]. Emerging evidence suggests mitochondrial dysfunction, endothelial injury, and cytokine-mediated myocardial suppression contribute to the progression from septic shock to cardiac standstill [11]. Unlike other cardiac arrests, SICA is predominantly associated with pulseless electrical activity (PEA) or asystole, which are non-shockable rhythms resistant to standard defibrillation, leading to significantly poorer outcomes [12]. This condition has a mortality rate approaching 70%, resulting from sepsis-related cardiac arrest [10]. Thus, early identification followed by specific treatment options should be included in the early medical literature.
This case report investigates a rare 63-year-old nursing home resident with multiple comorbidities, including CKD, diabetes, chronic anemia, and recurrent obstructive uropathy, who developed sepsis secondary to a complicated UTI caused by extended-spectrum beta-lactamase (ESBL)-producing E. coli. Despite early antibiotic therapy, fluid resuscitation, and emergency bilateral ureteral stenting, the patient deteriorated, developing new-onset atrial fibrillation (AFib) with rapid ventricular response, followed by PEA cardiac arrest. Successful advanced cardiovascular life support (ACLS) and intensive, multidisciplinary care ultimately stabilized the patient, allowing for recovery. It shows the highly multifactorial interplay of recurrent urinary tract infections with the sepsis-related systemic inflammatory response and cardiovascular compromise within high-risk populations necessitates early recognition of the clinical features, protocolized resuscitation, and several interventions to break the sepsis cascade. The case study explains SICA's pathophysiological mechanisms and time progression in a comorbid patient, revealing what might be done immediately to bolster risk stratification and evidence-based management of similar situations.
Case Presentation
A 63-year-old African American female nursing home resident was presented to the emergency department via EMS with a two-day history of progressive abdominal pain, nausea, vomiting, and lethargy. Past medical history is significant for type 2 diabetes mellitus with diabetic neuropathy, hypertension, schizoaffective disorder, obesity, acute transverse myelitis, overactive bladder, chronic pain, anemia of chronic disease, and bilateral osteoarthritis. She also reported increasing suprapubic discomfort (pain scale 9/10) and decreased oral intake. The patient expressed concerns about delays in hygiene care and urinary catheter maintenance at her nursing home, which she believed contributed to recurrent urinary tract infections. On arrival, she was hypotensive (103/58 mmHg) and tachycardic (HR: 110 bpm). Physical examination revealed a distended abdomen with moderate to severe tenderness in the suprapubic region. Laboratory workup demonstrated leukocytosis (WBC: 12.1 × 10³/μL), acute kidney injury (creatinine: 2.22 mg/dL), hypokalemia (3.1 mmol/L), and hyponatremia (sodium: 130 mmol/L). Urinalysis showed pyuria, hematuria, and nitrite positivity, while blood cultures grew Escherichia col. An initial Xray of the chest did not reveal any evidence of pulmonary etiologies (Figure 1).
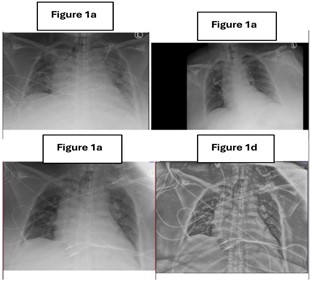
Figure 1: Chest X-ray. Initial Imaging (1a, 1b): A portable, one-view chest radiograph revealed cardiomegaly and aortic tortuosity without signs of pulmonary edema, airspace infiltrates, or pleural effusions, indicating no acute cardiopulmonary abnormalities. Follow-up Imaging (1c, 1d): A subsequent chest X-ray showed similar findings, reaffirming the presence of cardiomegaly without new or acute changes.
With clinical features consistent with complicated urinary tract infection (UTI) and sepsis, the patient was hemodynamically stabilized with 3.6 L of fluid bolus in the ED and was admitted to the medical-surgical floor...As the patient stabilized on vasopressors, persistent oliguria and acute kidney injury (AKI) with obstructive uropathy prompted a urology consultation. Renal ultrasound and CT imaging (Figure 2) revealed bilateral nephrolithiasis, including a 2.2 cm obstructing right ureteropelvic junction (UPJ) stone and an encrusted left ureteral stent placed for prior obstruction.
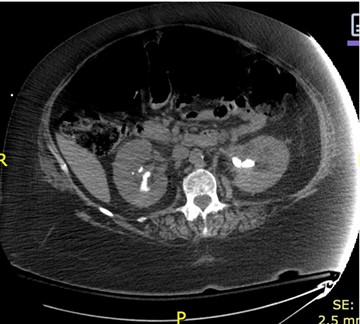
Figure 2: Interval placement of bilateral nephroureteral stents. A prior left nephroureteral stent is also demonstrated. 2.1 cm calculus at the right UPJ redemonstrated. Additional large bilateral renal calculi were demonstrated, similar to the prior examination. Mild bilateral hydronephrosis, slightly improved as compared to initial examination.
Ultrasound imaging (Figure 3) of the kidneys revealed bilateral nephrolithiasis without hydronephrosis. The kidneys appeared hyperechoic, suggesting potential medical renal disease.
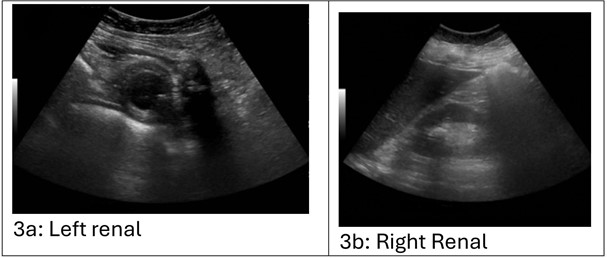
Figure 3a, 3b: Renal ultrasound demonstrating bilateral suggestive of obstructive uropathy. The left kidney is hyperechoic. The left kidney is standard in length, measuring 11.0 cm. No hydronephrosis. There are multiple renal calculi seen. For example, there is a 2.3 x 0.4 x 2.0 cm stone in the interpolar region of the kidney. There is a 1.3 x 0.4 x 0.8 cm stone in the inferior pole of the kidney. No focal parenchymal lesion. No perinephric fluid collection. No renal cysts. The right kidney is hyperechoic. The right kidney is standard in length, measuring 11.6 cm. No hydronephrosis. There are multiple renal calculi seen. For example, a 2.0 x 0.9 x 1.6 cm stone is in the right kidney's superior pole. There is a 1.6 x 0.5 x 1.4 cm stone in the interpolar region of the right kidney. No focal parenchymal lesion. No perinephric fluid collection. No renal cysts. Limited evaluation of the bladder secondary to decompression with Foley catheter. Partial visualization of distal double-J ureteral stents.
A few hours after admission, the patient's condition deteriorated with severe hypotension (70s/40s mmHg). The rapid response team (RRT) was activated, and the patient was placed in the Trendelenburg position while receiving a 1L NS bolus. Despite this intervention, the patient's blood pressure remained unstable. Midodrine was initiated following the completion of the fluid bolus. After a second 500cc fluid bolus and continued administration of midodrine, the patient's blood pressure improved to 95/53 mmHg. The pulmonology/critical care team was consulted, and they determined that ICU admission was necessary for continued pressure support and management of her critical condition. Nephrology was consulted to evaluate AKI secondary to septic shock and obstructive uropathy, attributing it to sepsis-induced ATN and possible post-renal obstruction. Management included strict volume control, electrolyte monitoring, really-dosed medication adjustments, and Foley catheter drainage. Renal function was monitored while awaiting urology intervention for stone removal.
In the ICU, the patient remained hypotensive despite fluid resuscitation, requiring vasopressor support with norepinephrine. The Pulmonary/Critical Care team managed pressor titration and oxygen therapy. The patient was maintained on 2L nasal cannula oxygen, with a plan to wean as tolerated. Given the concern for extended-spectrum beta-lactamase (ESBL)-producing E. coli in prior infections, Infectious Disease (ID) was consulted, and empiric cefepime was initiated. Due to ongoing bacteremia, antibiotics were later escalated to meropenem. Serial lactate levels showed initial elevation with a subsequent downtrend, suggesting improved perfusion. Telemetry revealed new-onset atrial fibrillation with rapid ventricular response (AFib with RVR) (Figure 4: Electrocardiogram (EKG) illustrating atrial fibrillation with rapid ventricular response (RVR), characterized by an irregularly irregular rhythm and absence of distinct P waves). The patient was tachycardic but asymptomatic for chest pain. Cardiology was consulted, and IV metoprolol was initiated for rate control. After the bleeding risk assessment, anticoagulation with apixaban was started. Serial ECGs demonstrated persistent AFib without ischemic changes or significant troponin elevation. The patient remained on vasopressors and continued sepsis management while further workup for obstructive uropathy progressed. Nephrology was consulted for acute kidney injury (AKI) secondary to septic shock and obstructive uropathy. Initial management included optimizing volume status, correcting electrolyte abnormalities, and adjusting excreted medications. A Foley catheter was maintained for urinary drainage. Creatinine peaked at 2.21 mg/dL with a BUN of 63.8 mg/dL, improving to 1.53 mg/dL and 57.6 mg/dL, respectively. Nephrology recommended continued conservative management.
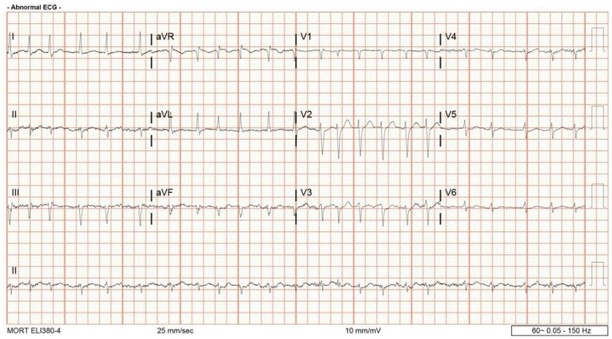
Figure 4: Electrocardiogram (EKG) illustrating atrial fibrillation with rapid ventricular response (RVR), characterized by: 1) Irregularly Irregular Rhythm: There is a complete absence of a repeating atrial or ventricular pattern, with varying R–R intervals, 2. Absence of Distinct P Waves: Instead of well-defined P waves, the baseline shows delicate undulations or fibrillatory waves, 3) Rapid Ventricular Rate: Many of the QRS complexes occur in close succession, indicative of a higher-than-normal heart rate.
Given the severity of obstruction and ongoing sepsis, urgent bilateral ureteral stent placement was planned. The patient underwent cystoscopy, bilateral ureteroscopy, and stent placement under general anesthesia. Intraoperative findings confirmed a severely encrusted left ureteral stent and obstructing right-sided UPJ stone. Stents were successfully placed bilaterally, and the stone burden was addressed with laser lithotripsy. No immediate post-procedural complications occurred. While the patient was being transported for a post-operative CT scan to assess for residual obstruction, she experienced a sudden cardiac arrest with pulseless electrical activity (PEA). Rapid response and Advanced Cardiac Life Support (ACLS) were initiated, and the patient received one dose of epinephrine and CPR, achieving the return of spontaneous circulation (ROSC) within minutes. Post-arrest evaluation, including CT head imaging, was unremarkable, and no clear etiology for the arrest was identified, though severe sepsis and hemodynamic instability were considered contributing factors. The patient remained intubated and sedated for continued critical care monitoring.
Following the patient’s post-operative cardiac arrest and persistent hemodynamic instability, infectious disease (ID) was consulted for further management of urosepsis. Blood cultures confirmed Escherichia coli bacteremia, with urine cultures revealing extended-spectrum beta-lactamase (ESBL)-producing E. coli. Given the patient's critical status and persistent leukocytosis, cefepime was escalated to meropenem for broader gram-negative coverage. The patient remained on vasopressor support, with daily lactate monitoring gradually improving. The patient also had complications of anemia due to acute kidney injury (AKI), and the patient had a hemoglobin of 8.6 g/dL with an estimated blood loss of 5 mL. The following day, hemoglobin declined, and two days post-procedure, it further dropped to 7.2 g/dL, followed by a further decrease to 6.8 g/dL the next day. Concurrently, MCV declined from 74.6 to 69.9 fL, indicating worsening microcytic anemia. The patient was transfused with 1 unit of packed red blood cells (PRBCs), leading to hemoglobin stabilization at 8.0 g/dL.
Renal function improved post-stent placement, suggesting resolution of the obstructive component. Over the next several days, vasopressors were successfully weaned off, and the patient was transitioned to oral midodrine for blood pressure support. She was extubated and transitioned to nasal cannula oxygen, with pulmonary/critical care overseeing her ventilator weaning. Fever curves, inflammatory markers, and repeat blood cultures were closely monitored, with eventual clearance of bacteremia. Sepsis resolved after a 14-day course of meropenem, allowing transfer from the ICU to the medical floor for further recovery. The patient remained hemodynamically stable, off vasopressors, and tolerating oral intake before discharge to a skilled nursing facility with oral antibiotics. Repeat imaging, as shown in Figure 5 below, is an Intraoperative fluoroscopic image confirming the successful placement of bilateral ureteral stents to relieve the obstruction and facilitate urinary drainage. This led to the resolution of hydronephrosis, and five weeks later, she underwent a planned bilateral ureteroscopy, laser lithotripsy, stone extraction, and stent exchange without complications. Intraoperative fluoroscopic images confirmed the placement of bilateral double-J ureteral stents, indicating a successful intervention for obstructing nephrolithiasis.
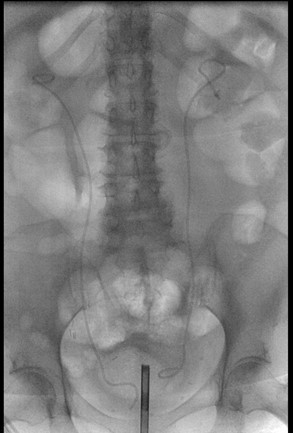
Figure 5: Intraoperative fluoroscopic images confirmed the placement of bilateral double-J ureteral stents, indicating successful intervention for obstructing nephrolithiasis.
Prompt multidisciplinary care facilitated early admission through ED, intensive care management, optimal sepsis management protocol (including early antibiotic treatment and source control), organized CPR/ACLS during the arrest, and successful discontinuation of vasopressors despite complications of anemia and post-arrest instability. Imaging included chest X-ray (cardiomegaly but with no acute changes) and CT/ultrasound (bilateral obstructing nephrolithiasis with hydronephrosis); timely interventions such as ureteral stenting were made possible because of these modalities. Serial ECGs tracked the transition from atrial fibrillation with rapid ventricular response to sinus rhythm in tune with individualized rate control. Social work facilitated safe nursing home discharge, which enabled continuity care for subsequent procedures, including stone removal and stent placement. The improved prognosis was confirmed by follow-up and laboratory and imaging studies. Renal function remained stable without recurrence of infections. This case demonstrates the lifesaving benefits of interdisciplinary and patient-centered management even in high mortality scenarios, such as Sepsis-Induced Cardiac Arrest (SICA).
Discussion
Sepsis is a deadly syndrome initiated due to a severe infection, causing abnormal immune reactions that result in inflammation, multiple organ failure, and cardiovascular collapse. This afflicts about 750,000 people each year in America alone and remains the top cause of ICU death around the world [8, 13]. Mayr et al. identified significant racial and health disparities in sepsis epidemiology, noting that Black populations and individuals with preexisting comorbidities experience higher incidence rates and face worse clinical outcomes compared to other groups [12]. Urinary tract infections (UTIs), a common cause of sepsis, can progress to urosepsis when bacteria such as Escherichia coli enter the bloodstream, triggering widespread inflammation [15]. Sepsis is associated with high mortality, particularly in older adults over 70 in developed nations [16]. In rare instances, sepsis leads to cardiovascular collapse with resultant sepsis-induced cardiac arrest (SICA), a less characterized and understudied syndrome that carries a mortality of greater than 70% [10]. With its predominance in non-shockable rhythms and poor resuscitation outcomes, further studies are required to strengthen early identification and treatment.
This case illustrates the life-threatening trajectory of obstructive uropathy secondary to a complicated urinary tract infection (UTI) in a high-risk patient. A 63-year-old nursing home resident with multiple comorbid conditions, including stage 3 chronic kidney disease (CKD), diabetes mellitus, recurrent UTI, and chronic anemia, a developed urosepsis caused by an ESBL-producing E. coli infection. Despite prompt broad-spectrum antibiotics, aggressive fluid resuscitation, and emergent bilateral ureteral stenting to relieve obstruction, she rapidly progressed to septic shock, new-onset atrial fibrillation (AFib) with the rapid ventricular response and subsequent pulseless electrical activity (PEA) arrest. Successful resuscitation required Advanced Cardiovascular Life Support (ACLS) and coordinated intervention by critical care, cardiology, nephrology, infectious disease, and urology teams. This clinical course underscores three crucial lessons: First, patients with CKD, diabetes, and anemia are at heightened risk for sepsis-induced cardiovascular collapse due to baseline metabolic and hemodynamic vulnerabilities. For instance, chronic renal disease in this patient predisposes her to cardiac dysfunction, as seen in cardiorenal syndrome [17]. Second, even timely antimicrobial therapy and source control may fail to prevent cardiac complications in the setting of profound inflammatory and oxidative stress, necessitating proactive cardiovascular monitoring (e.g., continuous telemetry, troponin trends) in septic patients with predisposing conditions [Rudinger]. Finally, multidisciplinary collaboration is essential to address the intersecting infectious, multiorgan pathologies that drive mortality in such cases [18].
The present case highlights the interplay between sepsis-induced cardiac dysfunction (SICA) and its pathophysiological underpinnings—including mitochondrial dysfunction and metabolic dysregulation—emphasizing the urgency of early recognition, protocolized multidisciplinary care, and translational research to address gaps in risk stratification and therapeutic innovation. SICA represents an advanced stage of sepsis-induced myocardial dysfunction characterized by profound hemodynamic instability, systemic inflammation, and acute myocardial depression [10, 11]. Unlike primary cardiac arrests, which are often caused by ischemic heart disease and present with shockable rhythms, SICA predominantly manifests as non-shockable rhythms such as PEA or asystole, making defibrillation ineffective and necessitating immediate ACLS intervention [19]. Complicated UTIs, particularly those associated with obstructive uropathy, prolonged urinary stasis, and recurrent bacterial colonization, sustain systemic inflammation, increasing the risk of cardiovascular collapse [3, 20].
The pathophysiology of SICA is driven by a cascade of inflammatory, metabolic, and microvascular dysfunctions that impair cardiac function. In gram-negative sepsis, endotoxins such as lipopolysaccharides (E. coli LPS) activate Toll-like receptor 4 (TLR4), triggering the release of pro-inflammatory cytokines, including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) [10,15].). These cytokines induce endothelial damage, vascular leakage, and systemic vasodilation, leading to hypotension and impaired myocardial perfusion [21]. Additionally, cytokine-mediated disruption of calcium homeostasis and mitochondrial dysfunction further reduces cardiac contractility, worsening cardiac output and exacerbating global hypoperfusion [22]. In this patient, the inflammatory cascade likely contributed to acute myocardial depression, evidenced by transient left ventricular dysfunction. The latest findings of Yu et al. 2025 recognize mitochondrial dysfunction and deranged energy metabolism as central mechanisms of sepsis-induced cardiac dysfunction, providing critical insight into potential targets of therapy against the prevention of this lethal complication in critically ill patients [23].
The concurrent presence of AFib further compromised hemodynamic stability, as atrial fibrillation in sepsis is associated with increased mortality due to impaired ventricular filling and loss of atrial contribution to cardiac output [24, 25]. This patient's arrhythmia, most likely the result of autonomic and inflammatory dysregulation electrolyte imbalance, highlights the importance of close cardiac monitoring and early control of rhythm in septic patients. The patient's anemia and CKD are the substrates for her risk of septic cardiac dysfunction. CKD comprises chronic inflammation, electrolyte imbalance, and fluid overload, all of which induce myocardial stress and increased susceptibility to arrhythmias [26]. In addition, anemia compromises oxygen delivery, increasing myocardial hypoxia and ischemic injury. In septic patients, the combined effect of anemia and inflammatory dysregulation increases myocardial stress, worsens cardiac function, and increases the risk of cardiovascular collapse. Effective anemia management through blood transfusion, erythropoiesis-stimulating agents, and close fluid balance monitoring is essential to improving outcomes in critically ill septic patients with CKD [6].
As described by existing literature, Urosepsis demands urgent multidisciplinary intervention, including early antibiotic administration and source control, to address its high mortality rates and prevent progression to life-threatening complications such as sepsis-induced cardiac arrest [Porat, Yang]. Notably, these complications may accelerate disease progression in elderly patients with severe urosepsis, as exemplified in this case [27]. This case underscores the critical role of early recognition, aggressive resuscitation, and multidisciplinary management in sepsis-induced cardiac dysfunction (SICA), aligning with evidence that protocolized, time-sensitive interventions—including ACLS principles, rapid source control (e.g., ureteral stenting), hemodynamic stabilization, and atrial fibrillation (AFib) rate control—reduce mortality in septic shock [9,10]. The success of this approach mirrors findings by existing studies emphasizing coordinated care between critical care and interprofessional teams to mitigate the consequence of sepsis-induced cardiac dysfunction, including end-organ hypoperfusion and secondary cardiac injury, mainly when guided by AFib strategies tailored to sepsis-induced hyperinflammatory states [25, 28].
Future research must prioritize SICA-specific biomarkers, such as mitochondrial dysfunction markers, to enable earlier diagnosis, as proposed, and refine treatment protocols through adaptive trials to address clinical heterogeneity [22]. Machine learning-driven predictive models, akin to frameworks for sepsis-associated AKI, could stratify high-risk patients. At the same time, standardized proactive cardiovascular evaluations (e.g., advanced hemodynamic monitoring) may enhance early intervention [29]. Raising clinical awareness of sepsis-induced cardiac arrest (SICA) and implementing tailored interventions for concurrent infectious, cardiac, and renal pathologies—especially in high-risk populations with comorbidities like CKD and diabetes—are critical to reducing mortality. Translating research on mitochondrial dysfunction into practice remains significant since the case highlights the need for such translation to prevent life-threatening cardiovascular morbidity [22]. It also emphasizes the need for individualized interventions against cardiorenal dysfunction to achieve optimal hemodynamic stability and enhance survival.
Conclusion
This case highlights the lethality of Sepsis-Induced Cardiac Arrest (SICA), particularly in high-risk patients with comorbidities like CKD and diabetes. Survival hinges on early sepsis recognition, prompt antibiotics, source control (e.g., ureteral stenting), and vigilant cardiac monitoring to detect dysfunction. The patient’s recovery underscores the necessity of multidisciplinary collaboration—integrating urology, infectious disease, cardiology, and critical care to address complex sepsis complications. SICA’s pathophysiology, driven by cytokine-mediated myocardial depression and metabolic instability, demands resuscitation strategies beyond standard ACLS, tailored to counteract inflammation and hemodynamic collapse. Comorbidities amplify sepsis severity, necessitating proactive management of conditions like anemia and CKD to mitigate cardiac strain. The limited literature on SICA in urosepsis calls for future research to identify biomarkers, refine predictive models, and establish evidence-based protocols for early intervention. Clinicians must prioritize cardiovascular assessment in septic patients, especially those with urinary obstructions or drug-resistant infections, to improve survival in this understudied, high-mortality complication.
References
- Bono MJ, Leslie SW, Reygaert WC. Uncomplicated Urinary Tract Infections. [Updated 2023 Nov 13]. In: Treasure Island (FL): StatPearls Publishing, 2025.
- Mancuso G, Midiri A, Gerace E, Marra M, Zummo S, Biondo C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens (Basel, Switzerland), 2023; 12(4): 623. https://doi.org/10.3390/pathogens12040623.
- Sabih A, Leslie SW. Complicated Urinary Tract Infections. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2025.
- Rishor-Olney CR, Hinson MR. Obstructive Uropathy. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2025.
- Liu G, Thomsen LE, Olsen Antimicrobial-induced horizontal transfer of antimicrobial resistance genes in bacteria: a mini-review. The Journal of antimicrobial chemotherapy, 2022; 77(3): 556–567. https://doi.org/10.1093/jac/dkab450.
- La Via L, Sangiorgio G, Stefani S, Marino A, Nunnari G, Cocuzza S, et al. The Global Burden of Sepsis and Septic Epidemiologia (Basel, Switzerland), 2024; 5(3): 456–478. https://doi.org/10.3390/epidemiologia5030032.
- Sakr Y, Jaschinski U, Wittebole X, Szakmany T, Lipman J, Ñamendys-Silva SA, et al. Sepsis in intensive care unit patients: Worldwide data from the intensive care over nations audit. Open Forum Infectious Diseases, 2018; 5(12): ofy313. https://doi.org/10.1093/ofid/ofy313.
- Gyawali B, Ramakrishna K, Dhamoon AS. Sepsis: The evolution in definition, pathophysiology, and management. SAGE open medicine, 2019; 7: 2050312119835043. https://doi.org/10.1177/2050312119835043.
- Guarino M, Perna B, Cesaro AE, Maritati M, Spampinato MD, Contini C, et al. 2023 Update on Sepsis and Septic Shock in Adult Patients: Management in the Emergency Department. Journal of clinical medicine, 2023; 12(9): 3188. https://doi.org/10.3390/jcm12093188.
- Habimana R, Choi I, Cho HJ, Kim D, Lee K, Jeong Sepsis-induced cardiac dysfunction: a review of pathophysiology. Acute and critical care, 2020; 35(2): 57–66. https://doi.org/10.4266/acc.2020.00248.
- Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Critical care medicine, 2007; 35(6): 1599–1608. https://doi.org/10.1097/01.CCM.0000266683.64081.02.
- Andrea L, Shiloh AL, Colvin M, Rahmanian M, Bangar M, American Heart Association's Get with The Guidelines®-Resuscitation Investigators, et al. Pulseless electrical activity and asystole during in-hospital cardiac arrest: Disentangling the 'nonshockable' Resuscitation, 2023; 189: 109857. https://doi.org/10.1016/j.resuscitation.2023.109857.
- https://www.thoracic.org/patients/patient-resources/managing-the-icu-experience/sepsis-severe-sepsis-and-septic-php
- Mayr FB, Yende S, Angus Epidemiology of severe sepsis. Virulence, 2014; 5(1): 4–11. https://doi.org/10.4161/viru.27372.
- Guliciuc M, Maier AC, Maier IM, Kraft A, Cucuruzac RR, Marinescu M, et al. The Urosepsis-A Literature Medicina (Kaunas, Lithuania), 2021; 57(9): 872. https://doi.org/10.3390/medicina57090872.
- Ibarz M, Haas LEM, Ceccato A, et al. The critically ill older patient with sepsis: a narrative Ann. Intensive Care, 2024; 14: 6. https://doi.org/10.1186/s13613-023-01233-7.
- H Mayow A. Atypical case of Cardiorenal syndrome. International Journal of Clinical Studies and Medical Case Reports, 2024; 38(1). https://doi.org/10.46998/ijcmcr.2024.38.000928.
- Gofron ZF, Aptekorz M, Gibas KW, Kabała M, Martirosian G. Retrospective Study of the Etiology, Laboratory Findings, and Management of Patients with Urinary Tract Infections and Urosepsis from a Urology Center in Silesia, Southern Poland Between 2017 and Medical science monitor: international medical journal of experimental and clinical research, 2022; 28: e935478. https://doi.org/10.12659/MSM.935478.
- Chalkias A, Spyropoulos V, Koutsovasilis A, Papalois A, Kouskouni E, Xanthos T. Cardiopulmonary Arrest and Resuscitation in Severe Sepsis and Septic Shock: A Research Model. Shock (Augusta, Ga.), 2015; 43(3): 285–291. https://doi.org/10.1097/SHK.0000000000000285.
- Silvani C, Bebi C, De Lorenzis E, Lucignani G, Turetti M, Jannello LMI, et al. Clinical and time-related predictors of sepsis in patients with obstructive uropathy due to ureteral stones in the emergency setting. World journal of urology, 2023; 41(9): 2511–2517. https://doi.org/10.1007/s00345-023-04513-w.
- Kamath S, Hammad Altaq H, Abdo T. Management of Sepsis and Septic Shock: What Have We Learned in the Last Two Decades? Microorganisms, 2023; 11(9): https://doi.org/10.3390/microorganisms11092231.
- Song J, Fang X, Zhou K, Bao H, Li Sepsis‑induced cardiac dysfunction and pathogenetic mechanisms (Review). Molecular medicine reports, 2023; 28(6): 227. https://doi.org/10.3892/mmr.2023.13114.
- Yu X, Gao J, Zhang C. Sepsis-induced cardiac dysfunction: mitochondria and energy Intensive Care Medicine Experimental, 2025; 13. https://doi.org/10.1186/s40635-025-00728-w.
- Margolin EJ, Wallace BK, Movassaghi M, Miles CH, Shaish H, Golan R, et al. Predicting Sepsis in Patients with Ureteral Stones in the Emergency Department. Journal of endourology, 2022; 36(7): 961–968. https://doi.org/10.1089/end.2021.0893.
- Induruwa I, Hennebry E, Hennebry J, Thakur M, Warburton EA, Khadjooi Sepsis-driven atrial fibrillation and ischaemic stroke. Is there enough evidence to recommend anticoagulation? European journal of internal medicine, 2022; 98: 32–36. https://doi.org/10.1016/j.ejim.2021.10.022.
- Zhou Y, Zhu Y, Wu Y, Xiang X, Ouyang X, Liu L, et al. 4-phenylbutyric acid improves sepsis-induced cardiac dysfunction by modulating amino acid metabolism and lipid metabolism via Comt/Ptgs2/Ppara. Metabolomics: Official journal of the Metabolomic Society, 2024; 20(3): https://doi.org/10.1007/s11306-024-02112-3.
- Porat A, Bhutta BS, Kesler S. Urosepsis. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2025.
- Yang WS, Kim Y-J, Ryoo SM, Kim Independent Risk Factors for Sepsis-Associated Cardiac Arrest in Patients with Septic Shock. International Journal of Environmental Research and Public Health, 2021; 18(9): 4971. https://doi.org/10.3390/ijerph18094971.
- Zarbock A, Nadim MK, Pickkers P, et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol, 2023; 19: 401–417. https://doi.org/10.1038/s41581-023-00683-3.

