Spinal Metastasis of Cerebral Glioblastoma
Faraj Kaoutar*, Hammoud Marouane, Hmamouche Oualid Mohammed, Lakhdar Faycal, Benzagmout Mohammed, Chakour khalid and El Faiz Chaoui Mohammed
Department Neurosurgery, Centre hospitalier universitaire HASSAN II, Morocco
Received Date: 18/03/2025; Published Date: 17/04/2025
*Corresponding author: Faraj Kaoutar, Department Neurosurgery, Centre hospitalier universitaire HASSAN II, Morocco
Abstract
Cerebral Glioblastoma (GBM) is one of the most aggressive malignant brain tumors, and spinal metastasis of cerebral glioblastoma via the cerebrospinal fluid (GBM) is a rare occurrence, with some reports suggesting a prevalence of 1% to 2%.
The goal of treatment is palliative, with no impact on overall survival, and the prognosis following a diagnosis of spinal metastasis remains poor.
We report the case of a 63-year-old patient who underwent surgical resection of a right temporo parietal glioblastoma, followed by adjuvant chemoradiotherapy according to the Stupp protocol. Four months after completing treatment, he developed a Frankel B paraplegia secondary to spinal metastasis of the glioblastoma, regarding T10 and L1, for which he underwent T10 laminectomy and histopathology revealed GBM and was subsequently managed with palliative radiotherapy.
We will discuss in this case report the pathway of metastasis, the clinical presentation, the treatment method and the prognosis.
Keywords: Spinal metastasis; Cerebral glioblastoma; Radiotherapy; Chemotherapy
Introduction
Glioblastoma (GBM) is the most common and most aggressive primary malignant glioma of the Central Nervous System (CNS), with a poor prognosis. The median survival following standard treatment with the Stupp regimen is approximately 14.6 months, and most patients ultimately succumb to tumor progression [1-2]. Tumor recurrence occurs in situ in 80% of cases, while 21.0% develop distant metastases, and a minority exhibit Metastatic Spinal Dissemination (MSD) [3]. Spinal metastases of intracranial GBM are exceedingly rare, with limited data available to accurately assess their incidence and prevalence. Existing reports estimate a prevalence of 1% to 2% among all patients diagnosed with cerebral GBM [4].
There are no established treatment guidelines for spinal metastases of GBM. The Stupp protocol, administered after the initial surgical resection of GBM, does not appear to confer a survival benefit in patients with spinal metastases.
Management is primarily palliative, aiming to alleviate pain and neurological deficits without significantly impacting overall survival. The prognosis following a diagnosis of spinal metastasis remains poor.
Spinal metastases should be strongly suspected in patients with a history of intracranial GBM who present with symptoms that cannot be explained by the primary lesion [5].
Case Report
A 64-year-old right-handed male patient with no significant medical history sustained head trauma. Following the injury, Computed Tomography (CT) scan of the brain was performed and incidentally revealed an intracranial lesion. On examination, the patient was conscious with stable hemodynamic and respiratory status, a Glasgow Coma Scale score of 15, and. Motor function was normal, with a muscle strength of 5/5 in all limbs, and sensation was preserved.
A subsequent brain MRI demonstrated a solid and cystic intra-axial lesion in the right temporo parietal lobe, with heterogenous contrast enhancement with surrounding diffuse edema that caused midline shift toward the left, suggestive of a glioma.
The patient then underwent a temporo parietal craniotomy with subtotal resection of the lesion (Figure 1), histopathological analysis confirmed the diagnosis of glioblastoma (GBM),
He subsequently received radiotherapy with adjuvant chemotherapy temozolamide according to the STUPP protocol.
Four months after completion of radiotherapy treatment, the patient developed progressive paraplegia over a period of 15 days and back pain, neurological evaluation revealed a Frankel B paraplegia with a muscle strength of 1/5, hypoesthesia, and a sensory level at the umbilicus.
A repeat MRI of the brain tumor recurrence at the primary site that cannot explain symptoms. (Figure 2).
magnetic resonance imaging (MRI) of the spine with contrast was performed, and had identify intradural nodular lesions at the level of T10 vertebra, another lesion along the cauda equina nerve roots at the level of L3 (Figure 3,4).
A T10 laminectomy with dural opening was performed, during which an infiltrative intramedullary lesion without a plane of demarcation with the spinal cord (Figure 5).
This lesion was partially resected and histopathological examination confirmed it to be a metastasis of glioblastoma, and the patient was subjected to palliative spinal radiotherapy.
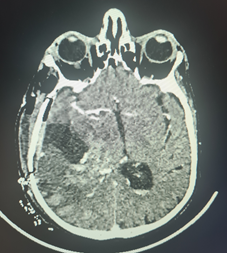
Figure 1: Post-operative Computed tomography of the brain, after glioblastoma resection.
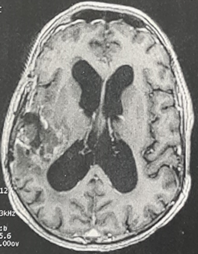
Figure 2: Axial T1-weighted brain magnetic resonance imaging (MRI) demonstrating a local tumor recurrence.
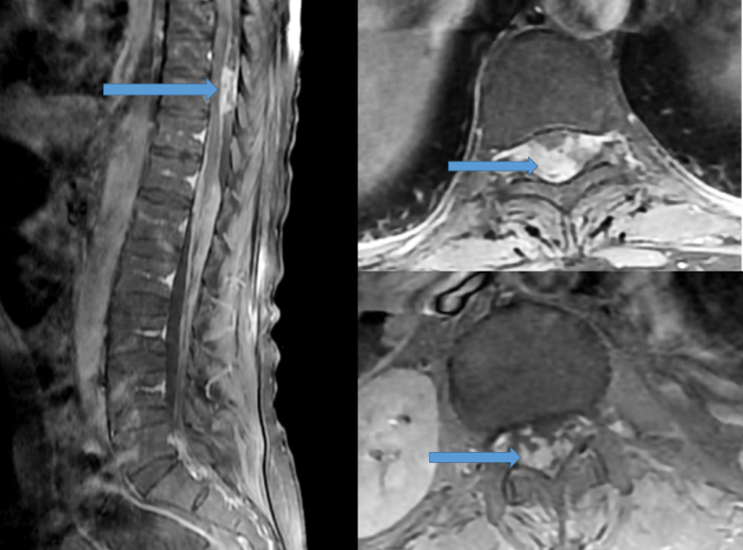
Figure 3: Sagittal and axial T1-weighted magnetic resonance imaging (MRI) of the thoraco lumbar spine with contrast showed nodal lesion at the T10 level (blue arrow).
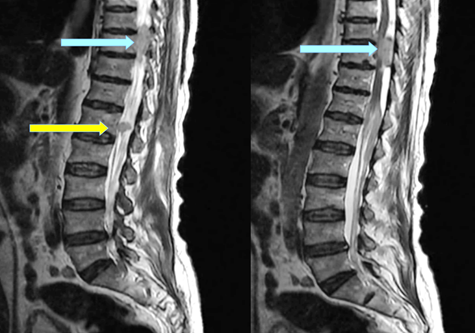
Figure 4: Sagittal post contrast T2-weighted magnetic resonance imaging of the lower thoracic spine and lumbar level showed nodal lesion at the T10 level (blue arrow), and second lesion in the cauda equine at the level of L1(yellow arrow).
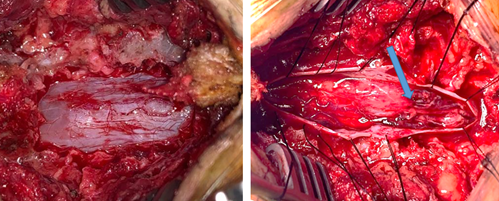
Figure 5: Intra operative view, demonstrate the intradural intramedular lesion (blue arrow).
Discussion
Glioblastoma (GBM) is a highly aggressive primary tumor in adults, composing of 12-20% of intracranial tumors and more than 50% of glial neoplasms, it is classified as a Grade 4 tumor according to the World Health Organization (WHO) classification of brain tumors [6].
Glioblastoma (GBM) was first described by Rudolph Virchow in 1863 [1], and represents the most common and most malignant tumor of the cerebral hemispheres, typically occurring between the ages of 40 and 60 years. The incidence in Europe and North America is estimated at 2 to 3 cases per 100,000 individuals per year, with 75% of patients succumbing to the disease within 18 months following diagnosis.
The incidence of spinal metastasis of cerebral glioblastoma is approximately 1.1% in the published literature. [7], in a serial autopsy study, approximately 25% of patients with intracranial GBM demonstrated spinal subarachnoid seeding; however, the exact incidence remains unknown [8].
The incidence of GBM metastasizing to the spinal compartment is likely higher than what is clinically detected, suggesting that spinal metastases have been underestimated in current clinical practice [9].
It is an infiltrating malignancy that recurs locally and it may spread along compact fiber pathways such as corpus callosum, optic irradiation, anterior commisure, and fornix or via cerebrospinal fluid (CSF) pathways. [10-11], cerebrospinal seeding (CSF) is present in approximately 15-25% of the cases of supratentorial GBM, while a higher incidence is seen in patients with infratentorial GBM [12].
When tumor cells infiltrate the Cerebrospinal Fluid (CSF) pathways, they can disseminate to any structure in direct contact with CSF, including the peritoneal cavity in cases where a ventriculoperitoneal shunt (VPS) is implanted.
The risk of spinal dissemination of tumor cells is increased following craniotomy, intraoperative ventricular entry, multiple resections, and in male patients [13]. Additional risk factors for CSF dissemination include ependymal invasion, ependymal fissuring due to hydrocephalus, and fragmentation of the tumor in contact with CSF.
Repeated tumor resection is associated with an increased risk of CSF dissemination, because of repeated tumor manipulation, more aggressive tumor cell types, and depressed immune function after radiotherapy and chemotherapy [14,15].
In a study on supratentorial gliomas in children conducted by Grabb et al., these factors were all found to be statistically significant contributors to the increased incidence of CSF dissemination [16].
The most frequent sites of spinal GBM metastases are the lower thoracic, upper lumbar, and lumbosacral regions. Spinal metastases commonly involve the nerve roots of the cauda equina, the root sleeves, and the fundus of the thecal sac, intramedullary metastases are exceedingly rare, with only a few cases reported in the literature [17].
Common symptoms of spinal metastasis include radicular pain, sensory loss, gait disturbances, and weakness. Patients may also experience pain in the lower back, interscapular area, and neck. As the condition progresses, paraparesis, quadriparesis, paraplegia, bowel or bladder dysfunction, and sexual dysfunction may develop [18]
Spinal MRI with gadolinium enhancement is currently the investigation of choice for diagnosing spinal metastasis. Lumbar CSF cytology is not routinely employed for this purpose due to the high incidence of false negative results.
The detection of tumor cells in the CSF is considered the most reliable criterion for diagnosing metastatic spinal dissemination (MSD) in GBM; but the chances of positive cytology are low. In a study by Chen et al., CSF cytology was found to be negative in all 14 observed cases [19].
Thus, whole spinal cord-enhanced MRI is currently considered the most effective diagnostic tool for metastatic spinal dissemination (MSD) in clinical practice.
There are no established guidelines for treatment of spinal metastasis from cerebral glioblastoma.
After the diagnosis of spinal metastasis, chemoradiotherapy is the primary treatment approach. Surgery may be considered for focal lesions, but chemoradiotherapy remains the only viable option for patients with diffuse or leptomeningeal disease.
The Stupp protocol, administered after initial brain surgery, does not appear to offer any survival benefit for patients with spinal metastases. The primary treatment goal is to alleviate pain and neurological deficits, without significantly impacting overall survival [1].
Unfortunately, the prognosis is very poor, with fatal outcomes. The time interval between the diagnosis of metastases and death ranges from 2 to 20 months, with an average survival of 2 to 3 months. Younger individuals tend to have a somewhat better prognosis [20-21].
Conclusion
Spinal metastases of glioblastoma (GBM) remain a rare but devastating complication, significantly worsening patient prognosis. Currently, there are no established treatment guidelines for managing these metastases, and the standard Stupp protocol does not appear to provide a survival benefit in these cases, treatment remains primarily palliative, focusing on symptom relief and quality of life
Further research is essential to better understand the mechanisms of CSF dissemination and to explore potential therapeutic strategies that could improve outcomes for this subset of patients.
References
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med, 2005; 352: 987-996.
- Brandes AA, Tosoni A, Franceschi E, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation With MGMT promoter methylation status. J Clin Oncol, 2009; 27: 1275-1279.
- Milano MT, Okunieff P, Donatello RS, et al. Patterns and timing of recurrence after temozolomide-based chemoradiation for glioblastoma. Int J Radiat Oncol Biol Phys, 2010; 78: 1147-1155.
- Tinchon A, Oberndorfer S, Marosi C, et al. Malignant spinal cord compression in cerebral glioblastoma multiforme: a multicenter case series and review of the literature. J Neuro-oncol, 2012; 110: 221-226.
- Birbilis, Theodossios A, Matis, Georgios K, Eleftheriadis Savvas G, Theodoropoulou Efthimia N, Sivridis Efthimios. Spinal Metastasis of Glioblastoma Multiforme: An Uncommon Suspect? Spine, 2010; 35(7): p E264-E269. DOI: 10.1097/BRS.0b013e3181c11748.
- Stark AM, Nabavi A, Mehdorn HM, Blömer U. Glioblastoma multiforme-report of 267 cases treated at a single institution. Surg Neurol, 2005; 63: 162–169.
- Ahmed Shaaban, Arun Babu R, Rasha G Elbadry, Rizq Haddad, Issam Al-Bozom, Ali Ayyad, et al. Spinal Metastasis of Cerebral Glioblastoma with Genetic Profile: Case Report and Review of Literature, World Neurosurgery, 2020; 143: Pages 480-489. https://doi.org/10.1016/j.wneu.2020.07.163.
- Raheja A, Borkar SA, Kumar R, Suri V, Sharma BS. Metachronous spinal metastases from supratentorial anaplastic astrocytoma. Asian J Neurosurg, 2015; 10: 60.
- Lawton CD, Nagasawa DT, Yang I, Fessler RG, Smith ZA. Leptomeningeal spinal metastases from glioblastoma multiforme: treatment and management of an uncommon manifestation of disease. J Neurosurg Spine, 2012; 17(5): 438–448.
- Mumenthaler M, Mattle H. Fundamentals of Neurology. Stuttgart, Germany: Georg Thieme Verlag, 2006: 94.
- Pohar S, Taylor W, Chandan VS, et al. Primary presentation of glioblastoma multiforme with leptomeningeal metastasis in the absence of previous craniotomy. Am J Clin Oncol, 2004; 27: 640–641.
- Arita N, Taneda M, Hayakawa T. Leptomeningeal dissemination of malignant gliomas: Incidence, diagnosis and outcome. Acta Neurochir, 1994; 126: 84–92. doi: 10.1007/BF01476415.
- Scoccianti S, Detti B, Meattini I, et al. Symptomatic leptomeningeal and intramedullary metastases from intracranial glioblastoma multiforme: a case report. Tumori, 2008; 94: 877-881.
- Tsung AJ, Prabhu SS, Lei X, et al. Cerebellar glioblastoma: a retrospective review of 21 patients at a single institution. J Neurooncol, 2011; 105: 555-562.
- Wright CH, Wright J, Onyewadume L, et al. Diagnosis, treatment, and survival in spinal dissemination of primary intracranial glioblastoma: systematic literature review. J Neurosurg Spine, 2019.
- Grabb PA, Albright AL, Pang D. Dissemination of supratentorial malignant gliomas via the cerebrospinal fluid in children. Neurosurgery, 1992; 30: 64–71.
- Hsu BH, Lee WH, Yang ST, et al. Spinal metastasis of glioblastoma multiforme before gliosarcomatous transformation: a case report. BMC Neurol, 2020; 20: 178. https://doi.org/10.1186/s12883-020-01768-3.
- Elsamaloty H, Zenooz NA, Mossa-Basha M. Glioblastoma Multiforme (GBM) of the conus medullaris with brain and brain stem metastases. Eur J Radiol Extra, 2006; 58: 59–62.
- Chen J, Shi Q, Li S, Zhao Y, Huang H. Clinical characteristics of glioblastoma with metastatic spinal dissemination. Ann Palliat Med, 2022; 11(2): 506-512. doi: 10.21037/apm-21-3387.
- Lindsay A, Holthouse D, Robbins P, et al. Spinal leptomeningeal metastases following glioblastoma multiforme treated with radiotherapy. J Clin Neurosci, 2002; 9: 725–728.
- Hubner F, Braun V, Richter HP. Case reports of symptomatic metastases in four patients with primary intracranial gliomas. Acta Neurochir (Wien), 2001; 143: 25–29.

