A Clinical Case of Primary Dystonia and Hypoceruloplasminemia Caused by Digenic Mutations in GNAO1 and CP Gene from Eastern India
Dipanwita Sadhukhan1, Amrita Karmakar2, Joydeep Mukherjee3, Bonny Sen3, Kartik Chandra Ghosh3, Soma Gupta2,4 and Arindam Biswas1,4,*
1Molecular Biology & Clinical Neuroscience Division, National Neurosciences Centre, Calcutta, Kolkata, India
2Department of Biochemistry, Nil Ratan Sircar Medical College, Kolkata, India
3Department of Neurology, Nil Ratan Sircar Medical College, Kolkata, India
4DBT-NIDAN Kendra, Nil Ratan Sircar Medical College, Kolkata, India
Received Date: 22/02/2025; Published Date: 27/03/2025
*Corresponding author: Dr. Arindam Biswas, M.Sc, Ph.D, National Neurosciences Centre, Calcutta Peerless Hospital (2nd floor), 360, Panchasayar, Kolkata 700094, India
Abstract
Mutations in GNAO1 (Gunanine Nucleotide-binding protein, Alpha-activating activity polypeptide O) are associated with rare autosomal dominant neurodevelopmental syndrome and movement disorders. Clinical manifestations appear very heterogeneous. Here, for the first time, we report an Indian male child with low serum ceruloplasmin level and 24-hour urinary copper without Kayser-Fleischer ring, manifesting sudden onset of dystonia. This study aims to identify the causal genetic variants (if any) associated with complex clinical manifestation by whole-exome-sequencing approach. Our WES data identified a de novo missense variant, c.644G>A (Cys215Tyr) in exon 6 of GNAO1 gene and novel 2 base-pair deletion in exon 1 of the Ceruloplasmin (CP) gene with maternal inheritance. Thus, our study implies the importance of Whole Exome Sequencing in hypoceruloplasminemia associated movement disorder to rule out Wilson’s disease.
Keywords: Dystonia; Hypoceruloplasminemia; GNAO1; CP; Mutations; Indians
Introduction
Neurodevelopmental disorder with or without variable movement or behavioural abnormalities is an autosomal dominant disorder, characterized by mild to severely impaired intellectual development and movement abnormalities [1]. It is a complex disorder with broad spectrum of clinical heterogeneity. Owing to the contributions from multiple genes in aetiology of neurodevelopmental disorder, molecular diagnosis is a prerequisite in order to explain the mixed phenotype as well to provide a proper treatment regimen. Since Whole Exome Sequencing (WES) is more potent than targeted gene sequencing for identification of missing / novel or all probable genetic variants associated with complex diseases, with respect to time and cost. Now a days, WES has become a common practice in the field of clinical neuroscience.
Here, we report an Indian male child patient who presented dystonia with normal MRI and EEG findings, lower 24-hour urinary copper & serum ceruloplasmin levels than the reference range, and absence of a Kayser-Fleischer ring. Since mixed phenotype challenged the accurate diagnosis, we aimed to apply the WES strategy to identify underlying causal genetic variations resulting in complex childhood neurological manifestations.
Case Report
The 6-year-old boy, born to non-consanguineous parents with no family history of neurological diseases, presented with an imbalance in the head and trunk with a tendency to fall backward, swaying to the right side with exaggerated posturing of both upper limbs and tremulousness in fingers and legs. He also had hyperkinetic dysarthria and postural tremor in both hands. The intensity of the tremor was more prominent while holding an object and eating. His hyperkinetic movement was not associated with intellectual disability (IQ score: 110). There was a history of neonatal sepsis, which required external oxygen support after birth. At the age of one-and-half years, he started facing difficulties while running. Abnormal involuntary posturing of lower limbs including imbalance, swaying to either side with posturing in the neck, and toe walking was observed. This insidious onset was found to be progressive. Additional manifestations like right eye uveitis associated with poor vision were also started at the age of two-and-half years of the subject. Despite such presentations, the boy was not subjected to any treatment up to his age of six years and four months. Suddenly, he developed a high-grade fever which sustained for consecutive five days. One week later, there was a sharp aggravation of his abnormal movements with the tendency to fall backward for which he was finally brought to the clinic for the first time from disease onset. Finally, he was subjected to trihexyphenidyl (3mg/day) treatment and responded well.
The brain magnetic resonance imaging (MRI) and EEG, NCV showed no signs of abnormality. As per the Unified Dystonia Rating Scale, the score was 11.5. Meanwhile, his investigations including complete hemogram, blood sugar, renal function tests, electrolytes, thyroid function test, and vitamin B12 were seemed to normal with lower urinary copper (11.7 µg/24 hours) and serum ceruloplasmin level (18.2 mg/dl). However, on slit lamp examination, the Kayser-Fleischer ring was found to be absent. At the same time, antinuclear antibody profile, serum VDRL, and HIV antibodies were found to be negative. Moreover, on neuropsychological evaluation using the Developmental Screening Test and Vineland Social Maturity Scale, no neuropsychological problem was identified.
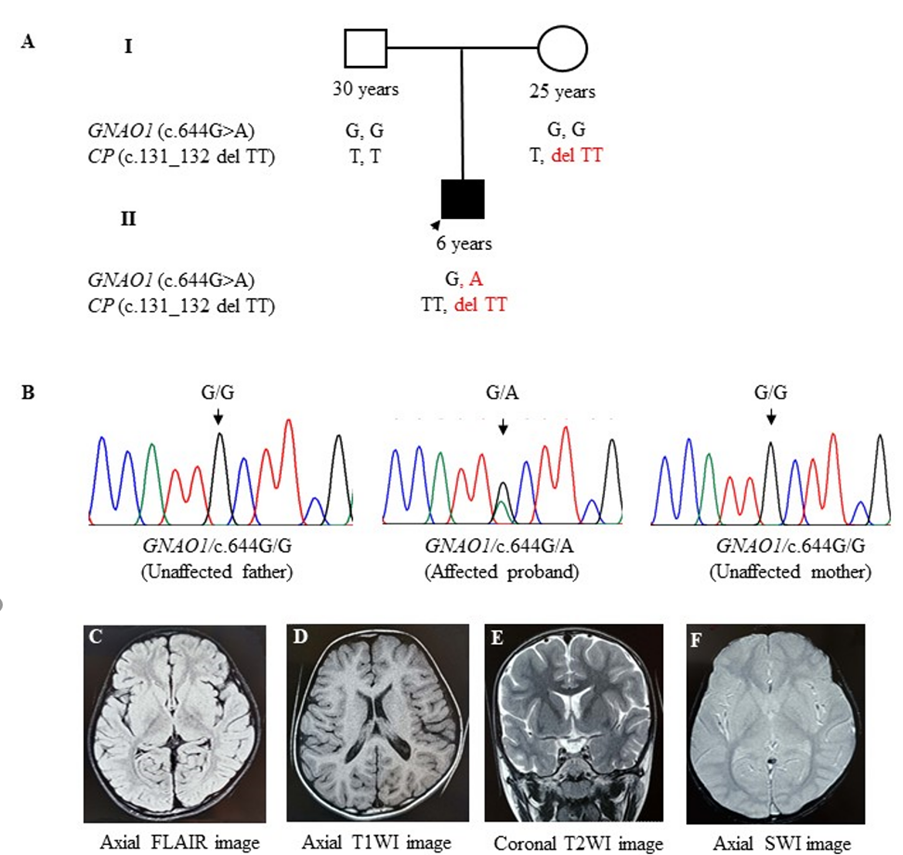
Figure 1: Screening of Cys215Tyr variant (c.644G>A) in GNAO1 gene and c.131_132 del TT in CP gene in a child affected with Neurodevelopmental disorder with movement disorder and hypoceruloplasminemia.
- The upper panel represents a 2-generation pedigree showing age and sex of each individual. The filled symbols indicate symptomatic GNAO1 and CP Segregation pattern of the GNAO1 and CP variant allele in family members.
- Chromatograms of the DNA sequence from the index case (II-1) showing the heterozygous condition of the c.644G>A of
Normal MRI Brain Axial Flair, (D) Axial T1W1, (E) Coronal T2W1 and (F) Axial SW1 images showing normal brain MRI.
Discussion
By doing WES, we identified causative variants in two disease-causing genes (GNA01 and CP) in a single infant included in our study. The GNAO1 gene encodes for the major neuronal G protein Gαo which transduces signals from numerous G protein-coupled receptors (GPCRs) such as D2 dopamine, μ-opioid, M2 muscarinic, or α2-adrenergic receptors. Mutations in this gene previously were found to be associated with childhood-onset epileptic encephalopathy or generalized dystonia with developmental delay [2]. The Cys215Tyr missense variant which was identified in our study, had previously been reported in a family showing a milder phenotype consistent with autosomal dominant inherited dystonia [3]. Such clinical manifestation was also reiterated in the GNAO1 [C215Y]/+ mouse model displaying distinct phenotypes like strong hyperactivity and hyper locomotion in a panel of behavioral assays, without signs of epilepsy [4]. Interestingly, the structural anomalies associated with this mutation observed in the mouse model were inconsistent with MRI findings from human subjects [4].
The other variant that was identified in this study subject was a 2 base pair deletion (c.131_132delTT) variant in exon 1 of the CP gene, resulting in a truncated peptide (Leu44HisfsTer4). This variant may explain the hypoceruloplasminemia phenotype without classical features of Wilson Disease which was genetically confirmed by the absence of mutation in ATP7B gene. Our present finding is consistent with a report by Lirong et al., 2009 where hypoceruloplasminemia-related movement disorder without KF-rings, exhibiting mild low serum CP was described as different from WD and also not associated with ATP7B mutation [5].
Therefore, in brief, two major phenotypes i.e., hyperkinetic movement with dystonic posture and low serum and 24-hour urinary copper level without Wilson disease phenotype can be explained by the variants described earlier. De novo mutations in the GNAO1 gene were initially reported in children with early infantile epileptic encephalopathies such as Ohtahara syndrome [2]. However, with growing evidence, neurodevelopmental disorder with involuntary movements (NEDIM) phenotype appeared as a major manifestation caused by the GNAO1 mutations without encephalopathy yet was severe enough to incapacitate the child to prevent independent sitting, standing, walking, or eating [6]. Moreover, the worsening of clinical condition by stress was reported to be a striking phenotype in this movement disorder while most of the patients show normal neuroimaging. Our case is in full accordance with such documented literature. In 2022, from India, another infant was reported with a different mutation (c.736G>A/p. Glu246Lys mutation) in GNA01 mutation predominately presenting severe hyperkinetic movements with the first episode of clinical seizure activity at the age of 7 years [7]. In an earlier report, the variants associated with movement disorders were classified as “gain of function” a mutation to be present outside the catalytic pocket i.e., between AA207 and AA221 of the protein [8]. With no exception, here also, the location of the mutation i.e., 215 positions were found to be associated with hyperkinetic movement disorders.
Conclusion
In conclusion, our study proves that dystonia along with hypoceruloplasminemia without K-F rings should be offered whole exome sequence to identify other genetic variants for accurate diagnostic, therapeutic, and prognostic purposes.
Credit authorship contribution statement: Dipanwita Sadhukhan, Soma Gupta, and Arindam Biswas were responsible for the concept, study design, and manuscript preparation. Joydeep Mukherjee, Bonny Sen, and Kartick Chandra Ghosh were responsible for the clinical diagnosis of study subjects. Amrita Karmakar was responsible for biochemical work and manuscript preparation. All authors read the draft, provided their inputs, and agreed on the final version of the manuscript.
Declaration of competing interest: The authors declare no conflicts of interest.
Acknowledgement: The authors thank the patients, family members, and healthy individuals who participated in the study.
Funding: Supported by grants from the Department of Science & Technology, Govt. of India, under the Cognitive Science Research Initiative Programme (DST/CSRI-P/2017/22), and Department of Biotechnology, Ministry of Science & Technology, Govt. of India (BT/NIDAN/01/05/2018).
Ethics statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee as well as the 1964 Helsinki Declaration and its later amendments.
Informed consent Informed consent from all the participants was received before clinical data and sample collection.
Data Availability Statement: The data described in this study are available from the corresponding author upon reasonable request.
References
- Parenti I, Rabaneda LG, Schoen H, Novarino G. Neurodevelopmental Disorders: From Genetics to Functional Pathways. Trends Neurosci, 2020; 43(8): 608-621. doi: 10.1016/j.tins.2020.05.004.
- Danti FR, Galosi S, Romani M, Montomoli M, Carss KJ, Raymond FL, et al. GNAO1encephalopathy: Broadening the phenotype and evaluating treatment and outcome. Neurol Genet, 2017; 3(2): e143. doi: 10.1212/NXG.0000000000000143.
- Wirth T, Tranchant C, Drouot N, Keren B, Mignot C, Cif L, et al. Increased diagnostic yield in complex dystonia through exome sequencing. Parkinsonism Relat Disord, 2020; 74: 50-56. doi: 10.1016/j.parkreldis.2020.04.003.
- Silachev D, Koval A, Savitsky M, Padmasola G, Quairiaux C, Thorel F, et al. Mouse models characterize GNAO1 encephalopathy as a neurodevelopmental disorder leading to motor anomalies: from a severe G203R to a milder C215Y mutation. Acta Neuropathol Commun, 2022; 10(1): 9. doi: 10.1186/s40478-022-01312-z.
- Lirong J, Jianjun J, Hua Z, Guoqiang F, Yuhao Z, Xiaoli P, et al. Hypoceruloplasminemia-related movement disorder without Kayser-Fleischer rings is different from Wilson disease and not involved in ATP7B mutation. Eur J Neurol, 2009; 16(10): 1130-1137. doi: 10.1111/j.1468-1331.2009.02733.x.
- Feng H, Sjögren B, Karaj B, Shaw V, Gezer A, Neubig RR. Movement disorder in GNAO1encephalopathy associated with gain-of-function mutations. Neurology, 2017; 89(8): 762-770. doi: 10.1212/WNL.0000000000004262.
- Satish S, Wilson R, Arunan S, Kalpana. A Rare Case of Movement Disorder with Developmental Delay [Clinical Phenotype of de novo Gnao1 03 mutation]: Case Report and Review of Literature. Indian J Neurol, 2022; 3(1): 107.
- Kehrl JM, Sahaya K, Dalton HM, Charbeneau RA, Kohut KT, Gilbert K, et al. Gain-of-function mutation in Gnao1: a murine model of epileptiform encephalopathy (EIEE17)? Mamm Genome, 2014; 25(5-6): 202-210. doi: 10.1007/s00335-014-9509-z.

