An Uncommon Case of Pacemaker Pocket Infection with Brucella Melitensis
Naveena Jagadeeshan*, Jayaprakash Shenthar, Vivek S Narayan Pillai, Mukund A Prabhu, Deepak Padmanabhan and Chollenhalli Nanjappa Manjunath
Fellowship in Electrophysiology and Advanced Adult Cardiology, Sri Jayadeva Institute of Cardiovascular Sciences & Research, India
Received Date: 25/01/2025; Published Date: 25/02/2025
*Corresponding author: Naveena J, Sri Jayadeva Institute of Cardiovascular Sciences & Research, Bannerghatta Road, Jayanagar 9th Block, Bangalore-560069, Karnataka, India
Abstract
We report a rare case of pacemaker pocket infection two years after implantation, with Brucella melitensis that was diagnosed by culture. Removal of the complete pacemaker system with leads, followed by treatment with doxycycline and rifampicin for 6 weeks resulted in cure.
Keywords: Device infection; Brucella melitensis infection; Pacemaker pocket infection
Introduction
Pacemaker and implantable cardioverter defibrillator infections, when not treated, leads to serious consequences. The aim is to identify the prevalent strains of the responsible bacteria to guide an effective therapy. The most common organisms causing pacemaker pocket infections are Staphylococcus species, Enterobacteriaceae, Pseudomonas aeruginosa, Streptococcus species, Enterococcus species and fungi [1].
Brucellosis is a zoonotic infection having varied clinical presentation and is transmitted to humans by direct contact with contaminated milk, milk products, meat and meat products [2]. Infection of a prosthetic device or implant by Brucella species is extremely uncommon. We report a case of pacemaker pocket infection with Brucella meletensis that was diagnosed by culture and treated appropriately.
Case Report
A 60-Year-old male patient who had undergone dual chamber permanent pacemaker implantation (Medtronic Relia DDD) two years ago for complete heart block presented with complaints of pain, ulceration, and purulent discharge from the site of pacemaker pulse generator (Figure 1). He was non-diabetic and his past history was unremarkable except for hypertension. On examination, patient was febrile and had tachycardia with normal blood pressure. Physical examination of the site revealed erosion of pacemaker with a boggy pocket and purulent discharge. Routine blood investigations revealed neutrophilic leukocytosis and sterile blood cultures. The patient underwent complete removal of the pacemaker system and leads, during which swabs were obtained from inside the pacemaker pocket and the surface of the pulse generator. The lead tips were also sent for culture. This was followed by wound debridement, irrigation of the pocket with povidone iodine, and antibiotic solution. The patient was empirically treated with intravenous ceftriaxone and gentamycin awaiting culture sensitivity report.
Gram stain of the wound swab showed few pus cells and no bacilli. The samples were cultured on blood agar, Macconkey’s medium, thioglycollate medium and Sabourauds dextrose agar and the plates were incubated at 37 degrees centigrade except blood agar which was incubated in an atmosphere of carbon dioxide. Forty-eight hours after incubation, translucent colonies were observed on blood agar, gram staining of which revealed gram-negative coccobacilli (Figure 2). This tested positive for oxidase and catalase tests and urease test was positive within 4 hours. The organism was confirmed to be Brucella melitensis using Vitek 2 compact (vitek@ 2 GN 21341 biomerieux). No growth was seen on Macconkey’s agar, Sabourauds agar even after 4 weeks of incubation. Subcultures from thioglycollate medium yielded translucent colonies which was again confirmed as Brucella melitensis. All microbiological work was carried out in a class 2 biosafety cabinet.
After the culture report, patient was switched over to oral doxycycline 100mg twice daily and rifampicin 450mg twice daily. Following seven days of antibiotics once the wound had healed, blood counts normalized, patient was afebrile and repeat blood cultures were negative; patient underwent successful re-implantation of a new pacemaker system on the opposite side. Patient was discharged on oral doxycycline and rifampicin for six weeks and continues to be well.
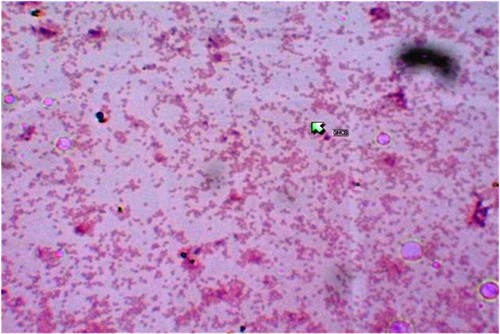
Figure 1: Pacemaker erosion with exteriorized lead and pulse generator.
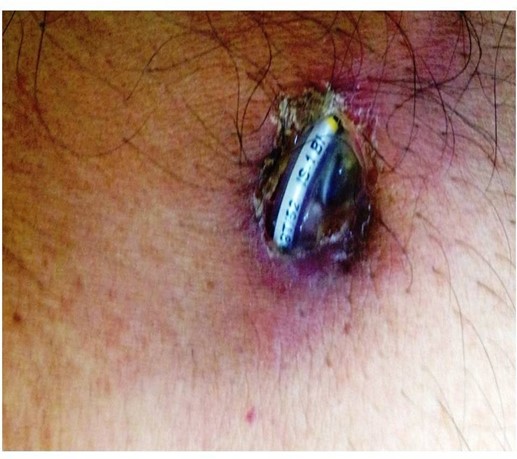
Figure 2: Gram Stain of growth on blood agar revealing gram-negative coccobacilli.
Discussion
The clinical presentations of cardiovascular implantable electronic device (CIED)-related infection can be broadly categorized into two groups: generator pocket infection and endovascular infection with an intact pocket [3,4]. The risk of device infection is estimated to be approximately 2% at 5 years after implantation [5]. About 60 – 80 % of infections are caused by either Staphylococcus aureus or coagulase-negative staphylococci. A variety of other bacteria and fungi are less commonly identified as causes of CIED infection [6,7]. Findings at the pocket site that are suggestive of infection include purulence, inflammatory changes, gelatinous material, loss or thinning of subcutaneous tissue, and poor capsule formation. Blood cultures are recommended in all suspected cases of infection, regardless of whether the patient is febrile or has other signs or symptoms of systemic infection. Blood samples should be drawn from different sites for at least two sets of cultures [8]. However, blood cultures may be negative despite infection, particularly in patients with pocket-site infection and in those given antibiotics shortly before blood samples are obtained for culture. It has been suggested that deep tissue specimens at the pocket site and lead tips should be obtained and cultured. In our case, these were obtained and when subjected to culture confirmed the diagnosis of infection with Brucella melitensis. The microorganisms that cause CIED infections may be acquired either endogenously from the skin of patients or exogenously from the hospital inanimate environment or from the hands of hospital workers. In support of endogenous acquisition, an association has been noted between the presence of preaxillary skin flora and the pathogens isolated from pacemaker infection8. Brucella infection of prosthetic materials including prosthetic joints, prosthetic valves, and ventricle septal defect patch has been reported [9,10]. Though Brucella species are primarily intracellular pathogens, they bind to extracellular matrix protein including laminin, fibronectin, and vitronectin. Prosthetic material implanted into humans become coated with these matrix proteins [11], thus explaining the ability of Brucella melitensis to infect devices and prostheses, even relatively long periods of time after insertion. Observations from several medical centers universally support complete removal of the device and the leads regardless of the extent of the infection to cure infection and reduce morbidity and mortality [12,13]. Complete removal of the prosthesis or the device followed by treatment with standard regimen for brucellosis results in good clinical outcome [14]. The recommended treatment of brucellosis is six weeks of oral doxycycline, combined with rifampicin for six weeks or parenteral streptomycin for two weeks [15].
Pacemaker pocket infection due to Brucella may be overlooked and misdiagnosed because of difficult diagnosis and the absence and lack of experience with laboratory testing. Alertness of medical staff is needed to recognize and diagnose the disease.
To the best of our knowledge, this is the first reported case of pacemaker pocket infection with Brucella meletensis in India.
Disclaimer
The material has not been previously published or submitted elsewhere for publication.
- All the authors had reviewed the article and agreed with its
- There is no conflict of interest of any of the
There has been no source of support in the form of grants, equipment or drugs.
References
- Camus C, Leport C, Raffi F, Michelet C, et Sustained bacteremia in twenty-six patients with a permanent endocardial pacemaker: assessment of wire removal. Clin Infect Dis, 1993; 17: 46-55.
- Smits HL, Kadri Brucellosis in India: Indian J Med Res, 2005; 122: 375-384.
- Gotuzzo E, Carillo C Brucellosis. In: Gorbach SL, Barlett JG, Blacklow NR, eds, Infectious disease 3rd ed. Philadelphia: Lippincott Williams & Wilkins 2004:1717- 24 Tarakj KG, Chan EJ, Cantillon DJ, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm, 2010; 7: 1043–1047.
- Sohail MR, Uslan DZ, Khan AH, et Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol, 2007; 49: 1851–1859.
- Silvetti MS, Fabrizio Drago, Giorgia Grutter, Antonella De Santis, Vincenzo Di Ciommo, Lucilla Ravà. Twenty years of paediatric cardiac pacing: 515 pacemakers and 480 leads implanted in 292 patients. Europace, 2006; 8: 530-536.
- Sohail MR, Uslan DZ, Khan AH, et al. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator Mayo Clin Proc, 2008; 83: 46-53.
- Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med, 2000; 133: 604-608.
- Da Costa A, Lelièvre H, Kirkorian G, Célard M, Chevalier P, Vandenesch F, et al. Role of the preaxillary flora in pacemaker infections: a prospective study. Circulation, 1998; 97: 1791–1795.
- Miragliotta G, Mosca A, Tantimonaco G, De Nittis R, Antonetti R, DiTaranto Relapsing brucellosis related to pacemaker infection. Ital Heart J, 2005; 6: 612-613.
- Cakalagaoglu C, Keser N, Alhan C, Brucella-mediated prosthetic valve endocarditis with brachial artery mycotic aneurysm J Heart valve Dis, 1999; 8: 586-590.
- Gristina Implant failure and the immune-incompetent fibro-inflammatory zone. Clin Orthop, 1994; 298: 106-118.
- Le KY, Sohail MR, Friedman PA, et Impact of timing of device removal on mortality in patients with cardiovascular implantable electronic device infections. Heart Rhythm, 2011; 8: 1678-1685.
- Pichlmaier M, Knigina L, Kutschka I, et Complete removal as a routine treatment for any cardiovascular implantable electronic device-associated infection. J Thorac Cardiovasc Surg, 2011; 142: 1482-1490.
- Abhay Dhand, Ross J. Implantable Cardioverter-Defibrillator infection due to Brucella melitensis: Case report and review of Brucellosis of Cardiac Devices. Clin Infectious Diseases, 2007; 44: e37.
- de la Fuenta A, JR Sanchez JR, Uriz J, Reparaz J, Lopez-Coronado JL, Moriones Infection of a Pacemaker by Brucella meletensis Tex Heart Inst J, 1997; 24: 129- 130.

